Background and overview[1]
4,4’-Biphenyldicarboxaldehyde is an important organic synthesis intermediate and one of the biphenyl derivatives. Biphenyl derivatives are widely used in medicine, polymer liquid crystals, dyes, pigments, etc. At present, the synthesis methods of aromatic formaldehyde products are divided into indirect electrooxidation methods, phase transfer catalysis methods and organic chemical synthesis methods. The main organic chemical synthesis methods include: chloromethylation method, acid halide method, gas phase or liquid phase selective oxidation method, hydroxy acid reduction method and other synthesis methods.
There are studies using biphenyl as raw material, bischloromethylation and hydrolysis to prepare 4,4’-biphenyl dicarboxaldehyde, with a total yield of only 30.8%. Or use biphenyl as raw material, undergo amine methylation, hydrolysis and salt formation to obtain 4,4′-diaminemethylbiphenyl hydrochloride, and then react with hexamethylenetetramine and then hydrolyze to obtain 4,4′ -Biphenyl dicarboxaldehyde, the overall yield is 57.8%. There are many synthesis methods for 4,4’-biphenyl dicarboxaldehyde, but the synthesis route is too long, the yield is low, and the production cost is high.
Preparation[1]
4,4’-biphenyl dicarboxaldehyde is prepared as follows:
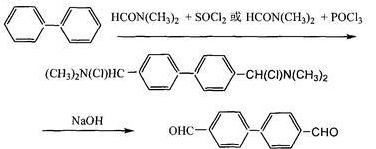
In a reactor equipped with a reflux condenser, add 4 mL of cyclohexane and 40.0 g (0.33 mol) SOCl2, stir and cool to about 10°C, and slowly add 22.0 g of N, N dimethylamide ( 0.3mol), a Vilsmeier coordination complex is formed after reacting for 0.5-2.0h; then 16.0g (0.1mol) of biphenyl solution dissolved in cyclohexane is added dropwise. After the dropwise addition is completed, the temperature is raised to 80-90°C. Maintain this temperature and stir the reaction for 8 hours, then cool to room temperature. In the ice-water cooling bath reactor, slowly add ice water dropwise to the reaction solution. After the dripping is completed, slowly add 30% sodium hydroxide solution under ice bath cooling until the pH reaches 5-6. Yellow flake crystals precipitate and are pumped. Filter, recrystallize the filter cake, and dry under vacuum to obtain 15.2g of 4,4′-biphenyl dicarboxaldehyde, m.p. 139-141°C. The purity measured by high liquid phase is greater than 98.0%, and the yield is greater than 70%.
Main reference materials
[1] CN201010000036.1 One-pot method for synthesizing 4,4’-biphenyl dicarboxaldehyde

 微信扫一扫打赏
微信扫一扫打赏

