Background and overview[1]
When the azobenzene polymer contains flexible molecular chains (such as alkyl chains), and the azobenzene molecules are connected to the molecular chains through covalent bonds, the azobenzene molecules can exhibit liquid crystal properties. When azo polymers are irradiated with light of appropriate wavelengths, this type of flexible material exhibits photocontrollable properties, such as photoinduced orientation, photochemical transformation, and photoinduced coordinated molecular motion. At present, there are many methods to prepare azobenzene liquid crystal polymers through covalent grafting. This type of azobenzene polymer is light-driven (such as UV light, blue-green light, polarized light, etc.). The main chain usually contains flexible molecular chains such as siloxanes, ethylenes, and acrylics. The photoresponsiveness of azo polymers is determined by both flexible segments and azo segments. 4-(4-Nitrophenylazo)-1-naphthol can be used to synthesize azobenzene polymers, especially methyl methacrylate azo polymers.
Apply[1]
4-(4-Nitrophenylazo)-1-naphthol can be used in the synthesis of azo monomers:
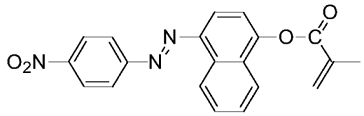
The specific steps are as follows: At 0°C, use methylene chloride as the solvent, add 0.01-1mol/L 4-(4-nitrophenylazo)-1-naphthol or 4-[(4-ethoxy Phenyl)azo]naphthol solution was added dropwise to methacrylic acid, 4-(4-nitrophenylazo)-1-naphthol or 4-[(4-ethoxyphenyl)azo] The molar mass ratio of naphthol to methacrylic acid is 2:1-1:5. Add 4-dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) to the system. The molar mass ratio of DMAP and DCC is 1:5-1:20; the reaction is first carried out at 0°C for 2-5 hours, after raising the temperature to 25°C, continue the reaction for 12-24 hours to obtain the azo product.
Main reference materials
[1] CN106188387-A photoresponsive methyl methacrylate azo polymer and its synthesis method

 微信扫一扫打赏
微信扫一扫打赏

