Background and overview[1][2]
1,5-Dibromo-2,4-dibromomethylbenzene can be used as a pharmaceutical synthesis intermediate, such as the preparation of organic electroluminescent device compounds: when the compound is used as a matrix material for fluorescent or phosphorescent emitters , this compound leads to very high efficiency and long life. This applies in particular if the compound is used as a matrix material for red or green phosphorescent emitters. When used in organic electroluminescent devices, the compounds according to the invention lead to high efficiencies and steep current/voltage curves at low operating voltages. When used as electron transport materials, the compounds according to the invention also lead to very good properties in terms of efficiency, lifetime and operating voltage of organic electroluminescent devices.
Preparation[1]
1,5-dibromo-2,4-dibromomethylbenzene can be used as a pharmaceutical synthesis intermediate, such as preparing compounds without 1h:
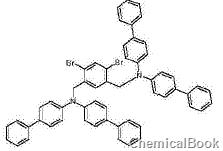
Dissolve 9.7g (0.243mol) of 60% NaH in mineral oil in 500ml of dimethylformamide under a protective atmosphere. 60 g (0.106 mol) N,N-bis(4-biphenyl)amine was dissolved in 500 ml of DMF and added dropwise to the reaction mixture. After 1 hour at room temperature, a solution of 242 mmol of 1,5-dibromo-2,4-dibromomethylbenzene in 500 ml of DMF was added dropwise. The reaction mixture was stirred at room temperature for 1 hour. Afterwards, the reaction mixture was poured onto ice and extracted three times with dichloromethane. The combined organic phases were dried over Na2SO4 and evaporated. The residue was extracted with hot toluene and recrystallized from toluene/n-heptane. Yield 80g (93%).
Apply[2]
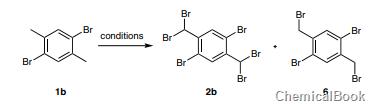
Bromination of 1b with NBS (4.4 equivalents) and AIBN (4 mol%) in refluxing ethyl acetate using a 100W incandescent lamp for 16 hours gave the symmetrical dibromide product 1,5-dibromo-2,4-di Bromomethylbenzene (6). Further increase in the amount of NBS to 8.8 equivalents and reaction time to 48 hours did not result in the formation of bis(dibromo) product 2b.
Main reference materials
[1] CN201280053788.1 Organic electroluminescent device
[2] Efficient Synthesis of Bis(dibromomethyl)arenes as Important Precursors of Synthetically Useful Dialdehydes

 微信扫一扫打赏
微信扫一扫打赏

