Background and overview[1]
3,5-bis(trifluoromethyl)phenyl isothiocyanate is mainly used as a pharmaceutical synthesis intermediate, such as the preparation of a series of thiourea compounds containing aromatic amine structures. This series of compounds has good properties with drug targets. The matching pattern has good inhibitory activity against tumor cell lines in vitro and can be used as an active ingredient to prepare tumor therapeutic drugs.
Structure
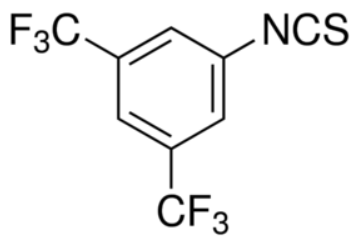
Preparation[1]
Synthesis of 3,5-bis(trifluoromethyl)phenyl isothiocyanate: Add 3,5-bis(trifluoromethyl)-4-chloroaniline (25.8g, 132.3 mmol), triethylenediamine (44.46g, 396.9mmol) and 135mL toluene, stir and dissolve at room temperature. Then add carbon disulfide (30.06g, 396.9mmol) dropwise within 2.0h. After the dripping is completed, keep the reaction at 15-25℃ for 8-10h. After the reaction is completed, filter with suction. The filter cake is rinsed once with 20mL toluene and dried to obtain a light Yellow powdery 3,5-bis(trifluoromethyl)-4-chloroaniline dithiocarbamate. Suspend the obtained 3-trifluoromethyl-4-chloroaniline dithiocarbamate in 200 mL of chloroform, start stirring and cool to -5-0°C. Slowly add 60 mL of chloroform dissolved with solid phosgene [bis (trichloromethyl) carbonate, BTC] (12.43 g, 42.0 mmol). After the dripping is completed, react in an ice bath for 1 hour, then at room temperature for 1 hour, and finally heat to Reflux and keep warm for 1.5-2h. After the reaction is completed, cool to room temperature, remove insoluble matter by suction filtration, and distill the filtrate under reduced pressure to obtain crude 3,5-bis(trifluoromethyl)phenyl isothiocyanate. After column chromatography (silica gel G, eluted with pure petroleum ether, concentrated under reduced pressure to obtain 23.5g of light yellow oily liquid, purity: 99.6% (GC), bp: 248-251°C, yield: 80.5%.
Main reference materials
[1]CN201510081559.6 Preparation methods and uses of diphenylthiourea compounds containing nicotinamide building blocks and their salts

 微信扫一扫打赏
微信扫一扫打赏

