Background and overview[1]
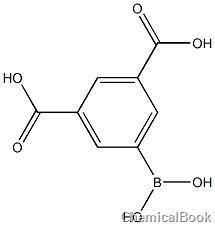
3,5-dicarboxyphenylboronic acid can be used as a pharmaceutical synthesis intermediate. It is an important intermediate for the synthesis of drugs, pesticides, liquid crystals, etc. Its application fields are constantly expanding and it has attracted people’s attention. In addition, aromatic carboxylic acids play an important role as ligands in coordination polymers and assembled supramolecules. Among them, aromatic polycarboxylic acids have a rigid structure, multiple coordination points, diverse coordination modes, and rich weak interactions between solvent molecules.
Apply[1]
3,5-dicarboxybenzeneboronic acid can be used to prepare N, N, N´, N´-tetraphenyl polycarboxylic acid aryldiamine compounds: under nitrogen protection, add to a 10 ml Schlek reaction tube (without A glass instrument commonly used in water anaerobic operation) add 1.0mmol4,4´-dibromobiphenyl, 3.0mmol bis(4-bromophenyl)amine, 0.10mmol palladium chloride, 0.10mmol di-tert-butyl (2 ‘,4′,6′-triisopropyl-3,6-dimethoxybiphenyl-2-yl)phosphine, 6.0mmol potassium tert-butoxide, and 5ml dioxane, replace the reaction tube with nitrogen 3 times, then heated to 110°C in an oil bath under magnetic stirring, and the reaction was refluxed for 48 hours. Cool to room temperature; then add 8.0mmol3,5-dicarboxybenzeneboronic acid, 0.10mmol palladium acetate, 0.10mmol2′-dicyclohexylphosphino-2,6-di-I-propyl-4-sulfonate- 1,1’-sodium biphenyl, 16.0 mmol cesium carbonate, and 200 mmol water; then heated to 110°C in an oil bath under magnetic stirring, and the reaction was refluxed for 48 hours. Cool to room temperature; add the filtered solid to 20 ml of water, adjust the pH of the aqueous solution to 1 with concentrated hydrochloric acid, stir at room temperature for 10 hours, filter, wash with ethanol, and dry to obtain product 3, with a yield of 65%. The mass spectrum (ESI) data of this product (CHNO) is 1144.15.
Main reference materials
[1]CN201611019660.XN, N, N`, N`? Synthesis method of tetraphenyl polyacid aryldiamine

 微信扫一扫打赏
微信扫一扫打赏

