Background and overview[1]
3,5-Dimethylbromobenzene is a very important fine chemical raw material. The traditional production process is complicated and the production conditions are harsh. According to current literature records, in the synthesis reaction of 3,5-dimethylbromobenzene, dimethyl sulfoxide (DMSO) is usually used as the oxidant and solvent for the bromination reaction, and hydrobromic acid is used as the bromination reagent to obtain 2-bromobenzene. -4,6-Dimethylaniline is then diazotized and hydrolyzed, and the final crude product is distilled to obtain the product. The bromination reaction of this process uses expensive DMSO as the solvent and cannot be recycled. Therefore, it inevitably has shortcomings such as low volume yield and high raw material cost. In addition, during the treatment process, excess solvent is washed away with water, and a large amount of waste solution not only pollutes It also harms the environment and keeps the cost of 3,5-dimethylbromobenzene high, making the market price of this product expensive.
Apply[2]
3,5-Dimethylbromobenzene is an important chemical intermediate. High-purity 3.5-dimethylbromobenzene can be used in the field of electronic chemistry and has broad market prospects. For example, to prepare bis(3,5-dimethylphenyl)phosphorus oxide, an intermediate of the bisphosphine ligand for the hydrogenation catalyst of the agricultural herbicide S-isopropylalachlor, first mix Mg, tetrahydrofuran and iodine. Add a small amount of 3,5-dimethylbromobenzene and heat to initiate the reaction. After the temperature of the mixed system rises to 50℃~60℃, lower the temperature and add a large amount of 3,5-dimethylbromobenzene, and heat the mixed system to 45℃~50℃. After reacting under the conditions until the reaction is completed, then lower the temperature and add diethyl phosphite dropwise, and stir the reaction until it is completed; then use hydrochloric acid to adjust the pH of the reacted system to 1, then add toluene to extract and separate the layers, and remove the organic layer to obtain a white solid , recrystallized from cyclohexane to obtain bis(3,5-dimethylphenyl)phosphorus oxide. The invention eliminates operating steps such as injection chromatography, increases production capacity, improves yield, reduces energy consumption, and reduces production costs.
Preparation[1,3]
Method 1: Preparation method of 3,5-dimethylbromobenzene, the method includes the following steps:
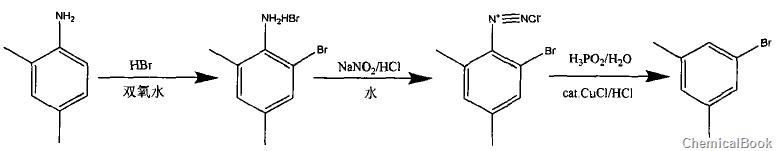
1) Mix and stir hydrobromic acid and 2,4-dimethylaniline, add hydrogen peroxide dropwise at a temperature of 35-45°C, complete the bromination reaction, and obtain 2-bromo-4,6-dimethyl Crude aniline bromate;
2) Add crude 2-bromo-4,6-dimethylaniline bromide into the hydrochloric acid solution, cool to 8~15°C, add 25% sodium nitrite solution dropwise, and control the temperature after the dropwise addition is completed 10~15℃, continue stirring for 6-12 minutes to obtain diazonium salt solution;
3) Take another reaction bottle, add 30% hypophosphorous acid, slowly add 9% cuprous chloride hydrochloric acid solution, lower the temperature to 10~15°C, and start dripping the above diazonium salt solution for 2-4 hours After the dropwise addition is completed, lower the temperature to 3-5°C and maintain it for 30-50 minutes, then raise the temperature to 20-30°C, react for 1.5-2.5 hours, add 50% sodium hydroxide solution, stir for 10-15 hours, and separate into static layers to obtain The finished product of 3,5-dimethylbromobenzene has a purity greater than 99.5% and a yield of 60.8%.
Method 2: A one-pot method for preparing 3,5-dimethylbromobenzene, which includes the following steps:
(a) Add 120g of glacial acetic acid and 36g of 2,6-dimethylaniline (0.3mol) into a 500ml three-necked flask, and then add dropwise sulfuric acid solution (containing 40g of 98% concentrated sulfuric acid and 100ml of water), keeping the temperature at Continue to add 48g of liquid bromine (0.3 mol) dropwise at 40°C, and continue to stir for 0.5 hours to complete the reaction. The dripping speed of the above sulfuric acid solution and liquid bromine is also the existing conventional parameter, as long as the reaction is not too intense and the material is flushed. ;
(b) Place the three-necked flask in an ice bath and let it drop to about 0℃; add sodium nitrite solution (containing 20.6g sodium nitrite and 60ml water) dropwise to it to keep it below 5℃ (Pay attention to controlling the heating speed and gas release speed to ensure that the generated nitrogen does not flush the material). After dripping (same as above), keep it warm and place it to form a diazo salt solution;
(c) Take 182g of hypophosphorous acid and cool it to 0°C, add 0.3g of copper sulfate to form a mixture, and then drop it into the reaction vessel of step (b) (i.e. dropwise into the diazonium salt solution, dripping parameter control requirements Same as above), after the dripping is completed, raise the temperature to 20°C and react overnight, a reddish-brown transparent organic layer will precipitate; let stand for layering;
(d) Take the organic layer and wash it with water and saturated sodium bicarbonate solution in sequence (can be washed multiple times respectively) to obtain the crude product (the measured purity is greater than 99%); then perform distillation to obtain a colorless transparent liquid, weight 52.3g (yield 95.13%), purity 99.9%.
Main reference materials
[1] Preparation method of CN200810230347.X3,5-dimethylbromobenzene
[2] CN201610999079.2 A one-pot method for preparing 3,5-dimethylbromobenzene
[3] Synthesis method of CN201410046420.3 bis(3,5-dimethylphenyl)phosphorus oxide

 微信扫一扫打赏
微信扫一扫打赏

