Background and overview[1]
2-Chloro-5-nitrobenzophenone is a ketone organic compound that can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
A preparation method of 2-chloro-5-nitrobenzophenone: add 1.0mol 2-chloro-5-nitrobenzoyl chloride (1.0mol ), 0.5 mol aluminum trichloride, 1 mol zinc chloride and 3000 ml methylene chloride, pass in nitrogen for protection, lower the temperature of the reaction solution to -20~-15°C, and then add 1.2 mol benzene dropwise. During the dropwise addition Control the temperature of the reaction solution to 20~-15°C. After the dropwise addition is completed, keep it warm and continue stirring for 2 hours. After the reaction is completed, slowly pour the reaction solution into 2000 ml of 0.5 mol/L hydrochloric acid (cooled to 0 to 10°C), keep the temperature at 0 to 10°C and continue stirring for 5 to 10 minutes. The water phase is extracted with 500 ml of dichloroethane. Combine the organic phases, wash once with 500 ml of saturated sodium carbonate solution, and then wash three times with 500 ml of water until neutral. Add 200 g of anhydrous magnesium sulfate to dry, and evaporate most of the methylene chloride. Add 500ml of a mixture of ethyl acetate and petroleum ether with a volume ratio of 2:1 to the remaining liquid to crystallize to obtain 2-chloro-5-nitrobenzophenone, and dry it to obtain 248.5g of product, with a yield of 95% and a purity of 99.5 %.
Apply[2]
2-Chloro-5-nitrobenzophenone can be used to prepare estrone and/or estradiol derivatives, such as the preparation of compounds of general formula II:
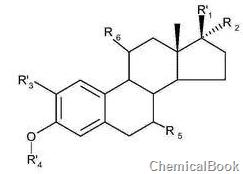
wherein R1 and R2 are independently hydrogen or hydroxyl or hydrocarbyl; or where R1 and R2 together are a double bonded oxygen; R3 is hydrogen; R’4 is nitrobenzophenone group, and R5 and R6 is independently hydrogen or hydroxyl or hydrocarbyl; the method starts from a compound of general formula I, wherein R1, R2, R3, R5 and R6 are as defined above, and R4 is hydrogen; wherein the compound of general formula I is reacted under basic conditions with 2-Chloro-5-nitrobenzophenone is reacted in the presence of an alkanol solvent, and the compound of formula II is crystallized directly from the alkanol solvent. Complexes of alkanols and compounds of general formula II obtained from the above methods, and methods of using the above methods or complexes.
Main reference materials
[1] CN201610768068.3 Preparation method of 2-chloro-5-nitrobenzophenone
[2] CN201110116385.4 Method for preparing estrone and/or estradiol derivatives

 微信扫一扫打赏
微信扫一扫打赏

