Background and overview[1]
4-Biphenylcarboxylic acid can be used as an intermediate in pharmaceutical synthesis, such as preparing biphenyldiazole derivatives. In vitro antibacterial activity tests show that this type of compound has strong antifungal and antibacterial activity.
Preparation[1]
Method 1: Preparation of 4-bibenzoic acid. Under argon protection, add 2.75g (13.8mmol) of 4-bromobenzoic acid, 2g (16.4mmol) of phenylboronic acid, 2.71g of K2CO3 (27.3mmol), and 0.2g of tetrakistriphenylphosphorus palladium to 40 mL of dioxane In cyclic solvent/water (10:1), the temperature was raised to 75°C, and TLC monitored the reaction to be complete after 5 hours. The reaction solution was cooled, filtered with suction, and the pH value was adjusted to 2-3. A large amount of white solid was generated. 2.30g of 4-biphenylcarboxylic acid, a white solid, was obtained by suction filtration with a yield of 84.9%. MS[M+H]+(m/z): 199.2.
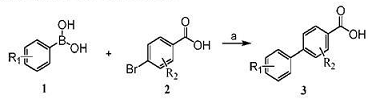 (R1=R2=H)
(R1=R2=H)
Method 2: Preparation of 4-bibenzoic acid: Add 1 mmol of p-phenylacetophenone (formula (1-16)), 0.1 mmol of I, and 0.1 mmol into a 25 mL glass tube equipped with a magnetic stirrer. Fe(NO)·9HO and 2 mL of DMSO, replace the air in the glass tube with oxygen and seal the glass tube. Then put the sealed glass tube into an oil bath preheated to 130°C, turn on the magnetic stirrer, and react After 12 hours, take out the sealed glass tube, wait for it to cool to room temperature, add water to the reaction solution to quench the reaction, then adjust the pH to about 11 with a sodium hydroxide solution with a concentration of 0.1 mol/L, wash three times with ethyl acetate, and water Use hydrochloric acid solution with a concentration of 0.1 mol/L to adjust the pH value to about 2, and then extract it three times with ether. Combine the three ether extracts, evaporate the ether under reduced pressure, and then perform column chromatography separation. Use ethyl acetate to separate the phase. The mixed liquid with a volume ratio of 1:25 to petroleum ether is used as the eluent. The eluate containing the target compound is collected, and the solvent is evaporated to obtain the product p-phenylbenzoic acid. The isolation yield is 81%.
Main reference materials
[1] CN201910018160.1 Biphenyldiazole derivatives and preparation methods and applications
[2] CN201810897789.3 A method for preparing arylformic acid using arylalkyl ketones as raw materials

 微信扫一扫打赏
微信扫一扫打赏

