Overview[1]
Me-trifluoromethylbenzoic acid is a chemical intermediate for medicines and pesticides, and is widely used in medicine, pesticides and other fields. At present, there are mainly the following preparation methods: 1) Using m-ditrifluorotoluene as raw material, heating with 30% fuming sulfuric acid and refluxing hydrolysis, cooling and adding ice water, filtering to obtain the product, yield: 66.1%. This preparation method has low yield and produces a large amount of waste acid, which does not meet the requirements of green and environmentally friendly production. 2) Use m-trifluoromethyl benzyl chloride as raw material, first chlorine it with chlorine under the action of initiator at 50-90°C, then use 2.5-10% sodium hydroxide to reflux and hydrolyze, neutralize and filter to obtain the product m-trifluoromethyl Benzoic acid, the total yield in two steps is 76.5-85.5%. Chlorine gas is used in this preparation method, which will be greatly restricted in actual use. Moreover, it is a two-step reaction and the total yield is not very high.
Preparation[1,3]
Method 1: A method for preparing m-trifluoromethylbenzoic acid, which uses m-trifluoromethylbenzyl chloride as raw material and oxidizes it with nitric acid with a concentration of 60-70% by weight at 100-110°C. Reaction, cooling and filtration, the m-trifluoromethylbenzoic acid product is obtained.
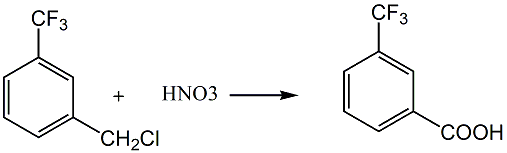
Method 2: Add 100g of m-(trifluoromethyl)benzyl chloride and 0.5g of initiator azobisisoheptanitrile into a 250ml three-necked flask, raise the temperature to 70°C, start to flow dry chlorine gas, and control the flow of chlorine The temperature is kept between 70-75°C. When the specific gravity of the reaction solution reaches 1.48-1.50, the chlorine flow is stopped, the residual chlorine is driven away, and the remaining chlorine is evaporated under a reduced pressure 2mmHg column at 53-55°C (reported by German patent DE2548970 (1997) Under a reduced pressure 11mmHg column, bp 87°C), 127g of 1-(trichloromethyl)-3-(trifluoromethyl)benzene with a content of 96.9% was obtained, and the yield was 91.8%. Then put 50g of the prepared 1-(trichloromethyl)-3-(trifluoromethyl)benzene and 1600g of 2.5% NaOH aqueous solution into a 3000ml three-necked flask, heat and reflux for 12 hours, and neutralize the pH of the reaction solution with 10% hydrochloric acid. to 4, a white precipitate precipitates, filtered, washed with a small amount of water, and vacuum dried at 60°C for 10 hours to obtain 32.4g of white powdery crude m-(trifluoromethyl)benzoic acid, content 97.3%, melting point 103-104°C, reaction The yield is 88.6%. After recrystallization of the crude product from CCl4, the melting point is 105.2-105.5°C (reported in the 2000-2001 edition of Aldrich Manual P.1662, the melting point of m-(trifluoromethyl)benzoic acid with a purity of 99% is 105-106°C).
Apply[2]
M-Trifluoromethylbenzoic acid can be used to prepare methyl m-trifluoromethylbenzoate: add 38.8g (0.2mol) of m-trifluoromethylbenzoic acid to the reactor, and use 155g of 1,2-dichloroethyl After the alkane is dissolved, add 13g of methanol, add 2g of cationic acid resin catalyst, stir, heat up to reflux reaction, and take out the water produced by the system in time. After the reaction is completed, the temperature is lowered and the catalyst is filtered out. The organic solvent is evaporated from the reaction material liquid under negative pressure. After the solvent is evaporated, the colorless and transparent product methyl m-trifluoromethylbenzoate is evaporated under negative pressure. The content is 99% and the yield is 95.5%. .
Main reference materials
[1] Preparation method of CN201310379816.5 m-trifluoromethylbenzoic acid
[2] CN201811627568.0 A synthesis method of m-trifluoromethylbenzoic acid methyl ester
[3] CN01132298.5 Preparation method of m-(trifluoromethyl)benzoic acid

 微信扫一扫打赏
微信扫一扫打赏

