Overview[1]
α-Bromo-3-acetoxyacetophenone is an organic intermediate that can be prepared from 3-acetoxyacetophenone through one-step bromination. Can be used to prepare α-(N-methyl-N-benzylamino)-3-hydroxyacetophenone hydrochloride. α-(N-Methyl-N-benzylamino)-3-hydroxyacetophenone hydrochloride is a key intermediate in the synthesis of phenylephrine. Phenylephrine is an adrenergic drug that has the effects of bronchodilation and constriction of nasal mucosal blood vessels. It can reduce congestion in the nasal cavity and sinuses, thereby relieving congestion and swelling of the nasal mucosa and alleviating symptoms of nasal congestion.
Preparation[1]
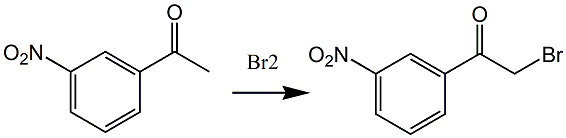
Preparation of α-bromo-3-acetoxyacetophenone:
Mix 3.36g (0.021mol) liquid bromine and 3.0g ethyl acetate to prepare liquid bromine solution A; add 11.4g ethyl acetate and 5.34g (0.030mol) into a 50mL three-necked bottle equipped with a thermometer and magnet ) of 3-acetoxyacetophenone to obtain a 3-acetoxyacetophenone solution; add 2 to 3 drops of bromine solution A to the obtained 3-acetoxyacetophenone solution, and continue after the reaction is initiated. Add the remaining liquid bromine solution A dropwise, and use an ice bath to control the internal temperature at 0 to 5°C. After adding the liquid bromine solution A, add 2.4 mL of hydrogen peroxide (0.021 mol) with a concentration of 0.3 g/mL, and keep in the ice bath. Control the internal temperature at 0 to 5°C for 8 minutes.
Pour the obtained reaction solution into a beaker containing sodium chloride solution (16g water + 1g sodium chloride) and stir for 15 minutes, then pour it into a separating funnel, shake, and let it stand to separate the water layer. The layer was back-extracted with 3.2g of butyl acetate, and the organic layers were combined and washed twice with 10 mL of saturated sodium chloride solution to obtain a butyl acetate solution of α-bromo-3-acetoxyacetophenone.
Apply[1]
For the preparation of α-(N-methyl-N-benzylamino)-3-hydroxyacetophenone hydrochloride:
To the butyl acetate solution of α-bromo-3-acetoxyacetophenone, add sodium carbonate solution (1.6g sodium carbonate dissolved in 11.2g water) dropwise to pH=8, add N-methyl dropwise 4.0g (0.033mol) of benzylamine liquid was added dropwise. During the dropwise addition, the internal temperature was controlled at about 25°C and maintained at this temperature for 1 hour.
Pour the obtained reaction system into a separatory funnel, add 14g of water, shake, let stand, and separate the liquids. The aqueous layer is back-extracted with 3.2g of butyl acetate. The organic layers are combined and washed twice with 10mL of saturated brine. Ice hydrochloric acid solution (obtained by adding 1g water to 9.0g 37.5wt% concentrated hydrochloric acid and cooling to 0~4°C) was used to back-extract the organic layer twice, during which the internal temperature was controlled at 12~18°C. Combine the acidic aqueous layer and wash it once with 3.2g of butyl acetate. Take the acidic aqueous layer and heat it to the internal temperature of about 40°C. Keep the reaction for 3 hours, then cool to the internal temperature of 0-5°C, precipitate the solid, and obtain the crude product by suction filtration.
In a 50mL three-necked flask, add 9g water, 16g acetone, 3.2g concentrated hydrochloric acid, 0.16g activated carbon and the above crude product, stir, raise the temperature to 50~60°C, decolorize for 1 hour, and filter while hot. Cool to 0 to 5°C, crystallize, filter with suction, rinse with ice acetone, and vacuum dry at 50 to 60°C for 8 hours to obtain 5.8 grams of finished product. The finished product is a white solid with a total yield of 70%, and its purity is greater than 99% as determined by HPLC.
Main reference materials
[1] [Chinese invention] CN201410657205.7 A preparation method of α-(N-methyl-N-benzylamino)-3-hydroxyacetophenone hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

