Background and overview[1-3]
2-Amino-4-cyanophenol is a phenolic derivative that can be used as an organic intermediate and can be prepared by one-step reduction of 2-nitro-4-cyanophenol.
Preparation[1-3]
Report 1,

Add 15g of 2-nitro-4-cyanophenol and 1g of 10% palladium on carbon into 120ml of methanol, add hydrogen, stir at room temperature for 8 hours, filter, collect the filtrate, and concentrate to obtain 13g of 2-amino-4 -Cyanophenol.
Report 2,
To 2-nitro-4-cyanophenol (1 g) and NH4Cl (163 mg) in ethanol (20 ml), THF (10 ml) and water (10 ml ) was added to the mixed solution of diatomaceous earth (5g) and reduced iron (1.7g), and then heated under reflux at 70°C. 30 minutes. The reaction solution was cooled to room temperature, diluted with EtOAc (200 ml), and filtered through celite. The solution was washed with saturated brine, the organic layer was dried over anhydrous MgSO4, filtered, and the filtrate was concentrated under reduced pressure to obtain 2-amino-4-cyanophenol (740 mg) as a light brown solid.
Report 3,
Dissolve 4-hydroxy-3-nitrobenzonitrile (0.5 g, 3.05 mmol) in ethanol (20 mL) and methanol (10 mL) and add Raney-Nickel at room temperature (1 mL, 10% aqueous solution), then add hydrazine monohydrate (0.296 mL, 6.09 mmcl). The mixture was stirred at room temperature overnight. The mixture was then filtered through Celite and concentrated by rotary evaporation. The residue was purified by chromatography (silica gel, n-hexane to ethyl acetate) to give 2-amino-4-cyanophenol (200 mg, 49%). 1H NMR: (DMSO-d6, 400 MHz) 4.99 (2 H, br.s), 6.74 (1 H, d, J 7.9), 6.82-6.89 (2 H, m), 10.23 (1 H, brs).
Apply[1]
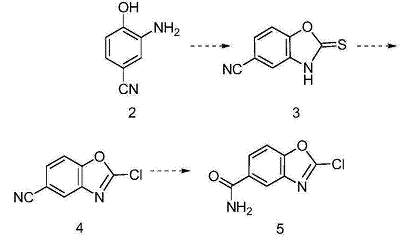
For the preparation of 2-chlorobenzo[d]oxazole-5-carboxamide. 2-Chlorobenzo[d]oxazole-5-carboxamide and related derivatives are widely used in medicinal chemistry and organic synthesis. 2-Amino-4-cyanophenol (intermediate 2) is subjected to ring closure reaction to obtain 3; 3 is subjected to chlorination reaction to obtain 4; 4 is subjected to hydrolysis reaction to obtain 2-chlorobenzo[d]oxazole-5 -Formamide (Intermediate 5), the synthesis route is as follows:
Main reference materials
[1] [Chinese invention] CN201710105657.8 Preparation method of benzo[d]oxazole derivatives
[2] From U.S. Pat. Appl. Publ., 20090076070, 19 Mar 2009
[3] From PCT Int. Appl., 2009037294, 26 Mar 2009

 微信扫一扫打赏
微信扫一扫打赏

