Background and overview[1]
Stearoylbenzoylmethane (SBM) is an important auxiliary heat stabilizer for plastics with a β-diketone structure. It can be compounded with a variety of stabilizers. Through the synergy between the components, it can Effectively improve the transparency and weather resistance of PVC products, and avoid precipitation and “zinc burning” during processing. It can not only reduce the early coloration of plastic products, but also improve the later stability. Compared with commonly used stabilizers, SBM has good thermal stability, chemical stability and light stability, is non-toxic and tasteless, and does not pollute products. Therefore, it is widely used in food and pharmaceutical packaging, such as mineral water bottles, oil bottles, and transparent sheets. and transparent films, etc. As the scope of applications continues to expand, the demand is increasing day by day.
Preparation[2]
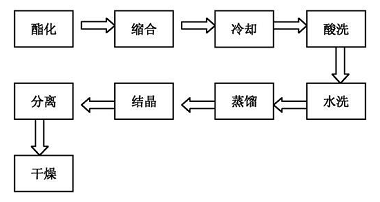
1) Synthesis of methyl stearate
Put stearic acid and methanol into a three-necked flask according to the set formula, and raise the temperature to 70°C. After the stearic acid is completely melted, drop in concentrated sulfuric acid and reflux for 2 to 3 hours. a. Esterification process conditions: stearic acid/methanol molar ratio 1:3~1:4; esterification reaction temperature at 65~70°C; reaction time 2~3 hours; b. water washing. Put the esterification liquid from step a into a separatory funnel and wash it 4-5 times with 200 ml of water until pH=7. Leave to separate the phases. The organic phase is crude methyl stearate, waiting for distillation; c. Distillation: Put the crude methyl stearate from step b into a three-necked flask, raise the temperature to 100°C, and distill under reduced pressure to remove low boiling points. Fraction to obtain methyl stearate;
2) Preparation of stearoylbenzoylmethane
Using methyl stearate and acetophenone prepared in step ① as raw materials and sodium methoxide as catalyst, the process includes the following steps:
a. Catalyst preparation
It is obtained by reacting metallic sodium with excess methanol. The reaction temperature is 20-50℃. After the metallic sodium reacts completely, the temperature is raised to 65℃. The excess methanol is recovered through distillation. The remaining part is the initiator sodium methoxide, which is used for the condensation reaction. ;
b.Condensation
Put 15g of sodium methoxide prepared in step a into a four-necked flask, add 150g of mixed solvent, stir and raise the temperature to 110°C, add 32.78g of methyl stearate prepared in step ①, when there is reflux in the reflux tube, add benzene 12g of ethanol, reflux and react at a temperature of 110°C to 120°C for 4 to 5 hours. The condensation yield is 88%.
c.Acidification
The temperature is 70℃~80℃. Add appropriate amount of deionized water to dilute it while stirring, neutralize the condensation product with 10% sulfuric acid to pH=6, continue stirring for 20~30 minutes, lower to room temperature, and let stand for layering. Remove the lower aqueous phase and wait for the organic phase to be washed with water; when the temperature drops below 60°C, add an appropriate amount of deionized water, increase the stirring speed, raise the temperature to 70°C to 80°C, and mix thoroughly without stratification. Then add 10% sulfuric acid dropwise to neutralize the sodium methoxide in the system, adjust the pH to 6, continue stirring for 20 to 30 minutes, lower to room temperature, let stand and separate, remove the lower aqueous phase, and wait for the organic phase to be washed with water;
d.Washed
Put the acidified organic phase in step c into a separatory funnel, cool to room temperature, wash with deionized water 4 to 5 times until the pH of the washing water = 7, let it stand for layering, remove the lower aqueous phase, and wait for the organic phase Solvent recovery;
e.Solvent recovery
Add the washed organic phase in step d into a three-necked flask, raise the temperature to 110°C, distill under reduced pressure, and collect the mixed solvent fractions to be recycled; crude stearoylbenzoylmethane is to be crystallized;
f. Crystallization
The crude stearoylbenzoylmethane after solvent recovery in step e is crystallized using industrial alcohol as the crystallization solvent at 0°C to 10°C for 12 to 18 hours; the crystallization yield is 95%. Pour industrial alcohol into the crude stearylbenzoylmethane flask after solvent recovery in step e while it is hot. After it is clear, use industrial alcohol as the crystallization solvent and crystallize for 12 to 18 hours at 0°C to 10°C;
g. Drying
The product crystallized in step f is filtered under reduced pressure, and after recovering the industrial alcohol, it is dried under normal pressure at a temperature of 30°C to 40°C to obtain the stearoylbenzoylmethane product.
Main reference materials
[1]Synthesis process of stearoylbenzoylmethane
[2]CN201110376321.8 Preparation method of stearylbenzoylmethane and its application in thermoplastic resin molding processing

 微信扫一扫打赏
微信扫一扫打赏

