Background and overview[1]
Amlodipine, chemical name is 3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(-2-chlorophenyl)-6-methyl-1 , 4-dihydro-3,5-pyridinedicarboxylate, is a dihydropyridine calcium antagonist developed by Pfizer in the United States in the 1990s. It is mainly used for the treatment of hypertension and angina pectoris as a treatment for hypertension. drug of choice, with sales ranking first among cardiovascular drugs in the world. Phthalyl amlodipine, as an important intermediate in the synthesis of amlodipine, is crucial to the further synthesis of amlodipine.
Preparation[1]
A synthesis method of phthalyl amlodipine, including the following steps:
(1) Synthesis of N-hydroxyethyl phthalimide
Add 200g phthalic anhydride and 600ml toluene into the reaction bottle equipped with a water separator, raise the temperature, add 100g ethanolamine dropwise, reflux the reaction until the reaction is complete, cool and filter, and dry at 80-90°C to obtain 247.6g N-hydroxyethyl Phthalimide, yield 96%.
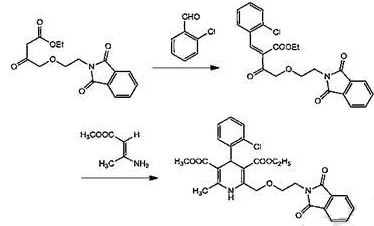
Synthesis of (2)ethyl-4-(2-phthalimidoethoxy)ethyl acetoacetate
a. Put 2400ml tetrahydrofuran into reaction bottle A, stir and cool to below -10°C, add 750g N-hydroxyethyl phthalimide and 345g NaH in sequence (while cooling, stir at low speed, N-hydroxy (The time interval between the addition of ethyl phthalimide and NaH and the start of the dropwise addition of ethyl 4-chloroacetoacetate is within 30 minutes); lower the temperature in the reaction bottle to below -10°C, and slowly add 732g4- When adding ethyl chloroacetoacetate, control the temperature below 0°C, and complete the dropwise addition within 1.5 to 2 hours; raise the temperature to 28 to 32°C, react for 5 to 6 hours, add 1500 ml of toluene, and lower the temperature to below 5°C. Obtain the toluene dilution of the reaction solution;
b. Add 1700ml toluene, 2800ml water, and 1178g acetic acid to reaction bottle B, and the temperature drops below 5°C; add the toluene dilution of the above reaction solution over 1 to 1.5 hours, and control the temperature below 10°C; stir for 10 minutes Then let it stand for 15 minutes, separate the liquids, and remove the water layer; the toluene layer was washed 5 times with 4% saline solution (2500ml each time). Anhydrous magnesium sulfate was added to the washed toluene solution for dehydration. After filtration, The toluene solution is concentrated under reduced pressure to remove toluene (the amount of toluene removed reaches more than 2700 ml, so that the toluene content is below the specified value) to obtain ethyl-4-(2-phthalimidoethoxy)ethyl acetoacetate. Toluene solution, the yield is 93%;
(3) Synthesis of phthalyl amlodipine
Continue to add 3000ml of isopropyl alcohol to the toluene solution of ethyl-4-(2-phthalimidoethoxy)acetoacetate, and add 564g of 2-chlorobenzaldehyde and 17.5g of it under stirring. Acetic acid, 24.8g piperidine; stir for 9 to 10 hours at 20 to 30°C, add 589g of β-aminocrotonate methyl ester, heat to 55 to 58°C, stir and react at 55 to 58°C, take a sample after stirring for 7 hours, and perform TLC Confirm that the reaction is complete, cool down to below 25°C, and add 3200 ml acetic acid; add seed crystals and cool to 0 to 5°C, stir at the same temperature for 5 hours, and crystallize; filter the crystals and wash with 800 ml of isopropyl alcohol. Washing is carried out twice. (400ml each time), and dried under reduced pressure to obtain 1611g of phthalyl amlodipine, with a yield of 76.1%.
Main reference materials
[1] CN201611244775.9 A kind of synthesis method of phthalyl amlodipine

 微信扫一扫打赏
微信扫一扫打赏

