Overview[1]
Mesitylenedine is an intermediate widely used in dyes, pesticides and other industries. The raw material for the synthesis of mesitylene is mesitylene, which exists in petroleum. With the realization of large-scale industrial production in my country, the output of mesitylene has continued to increase, so the development of its downstream products has received more and more widespread attention. Downstream products of mesitylene, such as trimesic acid, mesitylene, and M acid, are all important chemical products. Mesitylene is used as raw material to synthesize mesitylene. The nitration reaction of mesitylene is the key, which is directly related to the production cost and product quality.
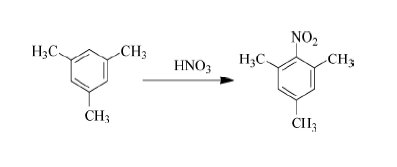
In 1987, the domestic Jinling Petrochemical Company was the first to successfully explore the synthesis process of using mesitylene as raw material, nitrification and reduction to obtain mesitylene and carry out industrial production. At that time, the annual output was 125 tons. Nitromesitylene is prepared by nitration with sulfuric acid and nitric acid respectively, and then catalytic hydrogenation and reduction is performed to prepare mesitylene. This method has a simple process and is easy for industrial production. In the following years, it became the mainstream process for preparing mesityleneamine.
However, its production process is accompanied by a large amount of waste acid and waste water, which increases the cost of subsequent environmental protection treatment. In addition, a large amount of dinitro by-products will be produced during the nitrification process, which requires distillation and purification in the later stage. Toluene has a high boiling point and requires a high distillation temperature, which greatly increases energy consumption and production costs. Using o-toluidine and methanol as raw materials, using γ-Al2O3-V, Cr, or Mg as the catalyst and high temperature (greater than 300°C) to produce mesityluidine through an alkylation reaction. The conversion rate of o-toluidine is The selectivities were 80% and 50% respectively. The operating temperature of this process is high, it is difficult to industrialize, and the conversion rate and selectivity are slightly poor.
Preparation[1]
1) First add 10g acetic acid into a 50mL constant pressure dropping funnel, then add 13.5g 98% nitric acid, and let it cool to below 25°C for later use. In a 250mL four-necked flask, add 24.5g of acetic anhydride and 24g of mesitylene in sequence, and add the prepared nitric acid solution dropwise while stirring at 20 to 25°C. After the dropwise addition is completed, keep the temperature at 20-25°C for 2 hours, then raise the temperature to 35-40°C and keep it for 2 hours. The sample is tested by liquid chromatography. When mesitylene is no longer detected, the reaction is terminated. The reaction equation is as follows:
2) Nitration reaction post-treatment
There are two main methods for post-treatment of nitrification reaction, water washing and distillation. Water washing method: After the nitration reaction is completed, add about 40g of water to the flask, raise the temperature to 65°C, separate layers while hot, and wash 2 to 3 times with 65°C hot water. The organic phase is nitromesitylene. Distillation method: After the nitration reaction is completed, the temperature is raised to 70~80°C, and then the acetic acid is removed by distillation under reduced pressure to obtain nitromesitylene.
Apply[2]
Mesityleneamine pure product is a colorless and transparent liquid. It changes color easily when exposed to air and light. The product is often light brown. Insoluble in water, soluble in organic solvents such as ethanol and ether. Mesitylene is an intermediate for dyes, organic pigments and pesticides. Its application examples are as follows: preparing a solvent blue 104 dye, which includes the following steps: (a) Add mesitylene to the reaction vessel, and add 1 , 4-dihydroxyanthraquinone, 1,4-dihydroxyanthraquinone leuco body, boric acid and catalyst, then the temperature is raised to 125~130°C, and the reaction is carried out for 15~18 hours to the end point; the mesityleneamine, 1,4- The mass ratio of dihydroxyanthraquinone, 1,4-dihydroxyanthraquinone leuco boric acid and catalyst is 12~18:3~5:1.5~2:0.8~1.5:1; the catalyst is selected from acetic acid, hydroxyl A mixture of one or more of acetic acid and salicylic acid; (b) cool the product of step (a) to 50~70°C, then add methanol for isolation; stir and then cool to 30~50°C, Just filter, wash and dry. This can improve the conversion rate and yield of products, reduce waste emissions, and help reduce environmental pollution.
Main reference materials
[1] Improvement of the synthesis process of mesityleneamine
[2] CN201710029347.2 An environmentally friendly preparation method of solvent blue 104 dye

 微信扫一扫打赏
微信扫一扫打赏

