Background and overview[1]
Benzofuran-6-carboxylic acid, also called 6-carboxylic acid benzofuran, is a benzofuran compound. This type of compound has a variety of good physiological activities, including anti-tumor, antibacterial, antioxidant and other effects, and has received widespread attention.
Preparation[1]
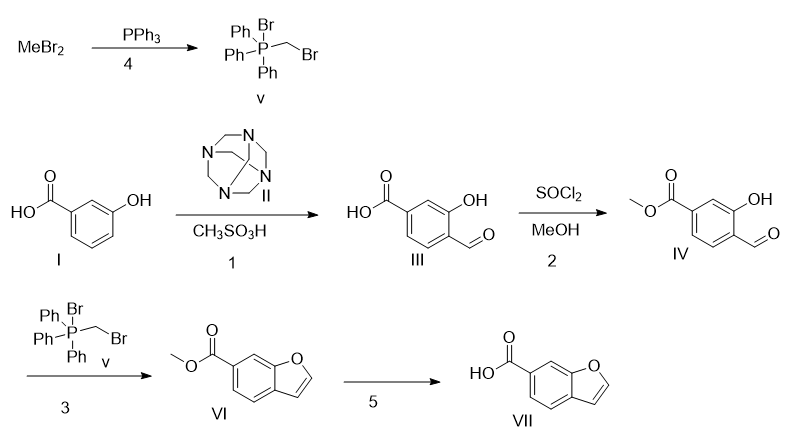
1. Add compound I (200kg, 1450mol, 1.0eq) and methanesulfonic acid (1000L) into a clean reaction kettle, stir and mix. Add compound II (305kg, 2170mol, 1.5eq) into the reaction bottle in batches, and control the reaction temperature within 80°C. After the addition is completed, heat at 80°C and stir for 3 hours. TLC analysis raw materials are consumed. Cool the reaction solution to 50°C, add 1500L pure water, and stir at room temperature for 1 hour after adding water. Use EA:MeOH (10:1) (500Lx6) to extract the product, combine the organic phases, and wash with saturated brine (300Lx2). Anhydrous sodium sulfate (20kg) was dried and filtered to remove sodium sulfate. The organic phase is extracted with 10% NaOH aqueous solution (300Lx4), the aqueous phase is combined, and the aqueous phase is adjusted to a pH value of about 3 to 4 using concentrated hydrochloric acid. Filter the solid to obtain wet product. After vacuum drying at 45°C, compound III was obtained as a white powdery solid (138.4kg, yield: 60%, purity: 94.2%). 1HNMR (400MHz, DMSO-d6): δ11.54(s,1H),10.29(s,1H),8.30(s,1H),7.65(d,J=9.4Hz,1H ), 6.80 (d, J = 8.7Hz, 1H).
2. Add compound III (10kg, 60mol, 1.0eq) and MeOH (100L) to the reaction bottle, then slowly add thionyl chloride (14.32kg, 120mol, 2.0eq), and raise the temperature to 60°C for 3 hours. TLC analysis showed that the reaction was complete. The reaction solution was concentrated. Add 100 LEA to dissolve, wash with 100 L pure water, dry with anhydrous sodium sulfate (1 kg) and filter. The organic phase was concentrated to obtain light yellow solid compound IV (10.6kg, yield: 96%, purity: 69.1%). 1HNMR (400MHz, DMSO-d6): δ11.05 (s, 1H), 10.37 (s, 1H), 7.75 (d, J = 8.0Hz, 1H), 7.58 (s, 1H) ), 7.48 (d, J = 8.0Hz), 3.87 (s, 3H).
3. Add compound IV (8.0kg, 44.41mol, 1.0eq), compound V (29.05kg, 66.61mol, 1.5eq) and THF (80L) into the reaction kettle under nitrogen protection. Cool the temperature to 0°C, add potassium tert-butoxide (8.26kg, 73.61mol, 1.65eq) in THF solution (1M) dropwise into the reaction kettle, control the temperature at 0~5°C, stir for 30 minutes, and continue to add tert-butoxide dropwise into the reaction kettle. Potassium butoxide (2.70kg, 24.06mol, 0.54eq) THF solution (1M) was heated at 0 to 5°C and stirred for 1 hour. TLC analysis: After the raw materials are consumed, 200L petroleum ether is added to the reaction solution to quench the reaction. Then add 200L petroleum ether and filter the insoluble matter. Concentrate to obtain an oily liquid, use 200L petroleum ether to disperse, and filter the reaction liquid. The filter cake is beaten with petroleum ether (200Lx2), the organic phases are combined, and concentrated to dryness. Continue to disperse 50L with petroleum ether, filter to remove insoluble matter, and beat the filter cake with petroleum ether (50Lx3). The organic phases were combined and concentrated to dryness to obtain 15.89kg of compound VI. The HPLC purity reached 95%, which was directly used in the next reaction. 1HNMR (400MHz, DMSO-d6): δ8.23(s,1H),10.37(s,1H),7.75(d,J=8.0Hz,1H),7.58(s,1H ), 7.48 (d, J = 8.0Hz, 1H), 3.89 (s, 3H).
4. Dissolve dibromomethane (148.5g, 0.85mol, 2.2eq) and triphenylphosphine (100g, 0.38mol, 1eq) in toluene (650mL) and react at 120°C for 24h. Cool down, filter, and wash the filter cake with petroleum ether. Collect the filter cake and spin it to dryness to obtain compound V (110 g, yield: 44%).
5. Add compound VI (15.89kg, 11.04mol, 1.0eq) and MeOH (30L) to the reaction bottle, stir to dissolve, add 27.65% NaOH aqueous solution (9L) to the reaction solution, stir at room temperature for 1 hour, and analyze the raw materials by TLC Consumed. Use 20L water to dilute the reaction solution, use 20L DCM to wash the reaction solution, use concentrated hydrochloric acid to adjust the pH value of the water phase to 2-3, filter the solid component, and drain it at 45°C to obtain white solid compound VII, that is, benzofuran-6-carboxylic acid (2.66 kg, yield: 33%). 1HNMR(400MHz, DMSO-d6)δ8.19(s,1H),8.12(s,1H),7.87(d,J=9.2Hz,1H),7.76(d,J= 8.0Hz,1H),7.07(s,1H).
Main reference materials
[1][Chinese invention] Preparation method of CN201910992537.3 benzofuran derivatives

 微信扫一扫打赏
微信扫一扫打赏

