Background and overview[1]
Methyl 3,4-dihydroxyphenylacetate can be used to prepare hydroxytyrosol. Hydroxytyrosol, whose chemical name is 3,4-Dihydroxyphenylethanol, is a hydroxyaromatic compound. Natural hydroxytyrosol rarely exists in free form in nature. It is only found to be obtained by enzymatic hydrolysis of oleuropein by β-glycosidase and esterase during the ripening process of olive fruits. Hydroxytyrosol is a polyphenolic natural product. The strong antioxidant properties and free radical scavenging ability of its o-diphenolic hydroxyl group make it have some biological activities that are beneficial to human health, such as anti-tumor, antibacterial, and anti-inflammatory. As well as the preventive effect on cardiovascular and cerebrovascular and sugar and lipid metabolism diseases.
Preparation[1]
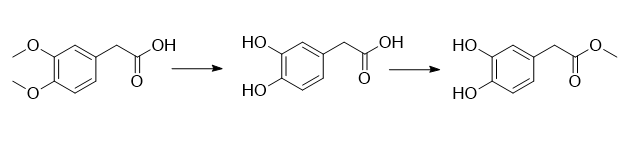
(1) Add 520g of dilute hydrochloric acid aqueous solution with a mass fraction of 10% into the reaction vessel, add 100g of 3,4-dimethoxyphenylacetic acid and 1.53g of glacial acetic acid under stirring at room temperature; complete the addition, at 80 React at ℃ for 6 hours; take a sample, and use HPLC to detect the complete reaction of the raw materials; lower the reaction solution to 60°C, distill the water under reduced pressure, add 200 mL of isopropyl ether to the remaining oil, and then freeze it in the refrigerator (0°C) overnight. A white solid precipitated and was filtered. The filter cake was washed with isopropyl ether and dried at 55°C to obtain pure 3,4-dihydroxyphenylacetic acid, 83.5g, with a yield of 96.9%.
(2) Add 100 mL of methanol to a reaction vessel, add 83.5 g of 3,4-dihydroxyphenylacetic acid with stirring at room temperature, and after it is completely dissolved, add 4.98 g of concentrated hydrochloric acid (mass fraction: 36% ); after the addition is completed, react at 56°C for 1 hour; take a sample, and detect the complete reaction of the raw materials by HPLC; lower the reaction solution to room temperature, concentrate under reduced pressure to recover methanol, add 100 mL of water to the remaining oil, and then use methyl tert-butyl Extract with ether, collect the organic layer, dry it, and concentrate to dryness to obtain 3,4-dihydroxyphenylacetic acid methyl ester, 89.5 g, with a yield of 98.4%.
Main reference materials
[1]CN201910495320.1 Preparation method of hydroxytyrosol

 微信扫一扫打赏
微信扫一扫打赏

