Background and overview[1]
2-Bromo-6-(trifluoromethoxy)benzoic acid can be used as a pharmaceutical synthesis intermediate. It can use 2-amino-6-(trifluoromethyl)benzoic acid as the starting material for the reaction. It is prepared by reacting sodium nitrate with potassium bromide and can be used to prepare pyrazole amide derivatives, which have certain uses in the treatment of various diseases, conditions or disorders such as allergic disorders, inflammatory disorders and immune system disorders.
Preparation[1]
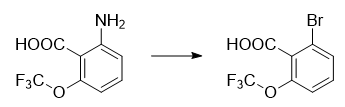
2-Bromo-6-(trifluoromethoxy)benzoic acid is prepared as follows: In an ice-water bath (internal temperature is 0°C), add 2-amino-6-(trifluoromethyl)benzoic acid (10 g , 48.7 mmol, Apollo) to a suspension in water (43 ml) was added 7 mL of concentrated sulfuric acid (specific gravity 1.84, 131 mmol). To this slight suspension was added dropwise an ultrafree-flowing solution of sodium nitrite (3.76 g, 54.5 mmol, Aldrich) in ice-cold water (20 ml). The suspension was stirred in an ice-water bath for 20 minutes. To the mixture was added a solution of potassium bromide (12.14g, 73.1mmol, Acros) in ice-cold 1M sulfuric acid (30ml). The mixture was allowed to warm to ambient temperature and then heated to 80°C under nitrogen for 45 minutes. Allow the mixture to cool to ambient temperature. The mixture was partitioned between ethyl acetate (300 ml) and water (300 ml). Separate each phase, wash the organic phase with brine (50ml×2), dry (MgSO4), filter, and remove the solvent in vacuo to obtain the title compound 2-bromo-6-(trifluoromethoxy) Benzoic acid, dark brown solid (16.7g).
Main reference materials
[1] WO2010122089 – N-PYRAZOLYL CARBOXAMIDES AS CRAC CHANNEL INHIBITORS

 微信扫一扫打赏
微信扫一扫打赏

