Background and overview[1]
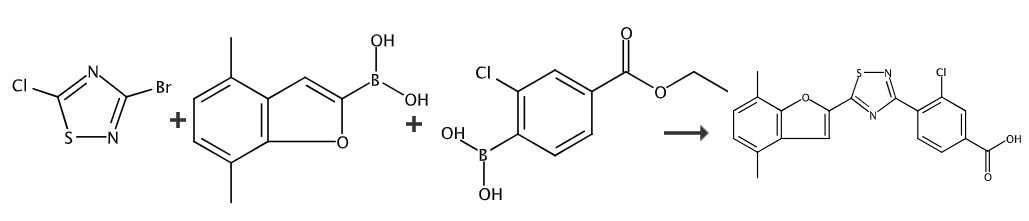
Ethyl 4-boron-3-chlorobenzoate can be used as a pharmaceutical synthesis intermediate and applied in Suzuki coupling reaction. CN107207488 reports that ethyl 4-boron-3-chlorobenzoate can be used to prepare 3-chloro-4-(5-(4,7-dimethylbenzofuran-2-yl)-1,2,4-thiodi Azol-3-yl)benzoic acid, a compound that selectively activates retinoic acid receptor beta (RARβ) (eg, RARβ2) in vitro or in vivo.
Application examples[1]
Preparation of methyl 3-chloro-4-(5-(4,7-dimethylbenzofuran-2-yl)-1,2,4-thiadiazol-3-yl)benzoate: Add 3-bromo-5-(4,7-dimethylbenzofuran-2-yl)-1,2,4-thiadiazole (0.323mmol) and 4-boron-3-chlorobenzene into the sealed tube. Ethyl formate (0.388mmol), K3PO4.H2O (0.647mmol), Pd (Ph3P)4 (0.03mmol), DMF (2mL) and H2O (0.5mL), the mixture was heated in a microwave reactor at 130°C Heat for 15 minutes. The mixture was cooled to RT and partitioned between DCM (10 mL) and H2O (3 mL). The organic solution was washed with water (3 mL), passed through a phase separation column and concentrated in vacuo. The residue was purified by silica gel chromatography, 0-10% EtOAc in isohexane to give 3-chloro-4-(5-(4,7-dimethylbenzofuran-2-yl)-1,2 ,4-thiadiazol-3-yl) methyl benzoate.
Main reference materials
[1]CN107207488 Bicyclic heteroaryl-heteroaryl-benzoic acid compound as a retinoic acid receptor B (RARΒ) agonist

 微信扫一扫打赏
微信扫一扫打赏

