Background and overview[1-2]
3-Amino-4-chlorobenzonitrile is a pharmaceutical intermediate. It can be nitrated from p-chlorobenzonitrile to obtain 4-chloro-3-nitrobenzonitrile, and then reduced to obtain 3-amino-4-chlorobenzonitrile. Carbonitrile. There are reports in the literature that 3-amino-4-chlorobenzonitrile can be used to prepare 2-amino-4-cyano-3′,4′-difluoro-1,1′-biphenyl, and fluorinated benzidine is an important class of The intermediates are widely used in industrial production fields such as pesticides and medicines.
Preparation[1-2]
Step 1,

Add 5g of p-chlorobenzonitrile to 30g of concentrated sulfuric acid, start stirring, cool to 0°C in an ice-salt bath, add 5.7g of potassium nitrate in batches, complete the addition, stir for 2 seconds in an ice-salt bath hours, stir at room temperature overnight, and detect the reaction is complete by TLC plate. After the reaction is completed, pour the reaction solution into ice water (200 ml), stir for half an hour, filter with suction, wash with ice water until neutral, and dry at 70°C. 6.3 g of 4-chloro-3-nitrobenzonitrile was obtained as an off-white powder, with a yield of 95%.
Step 2,
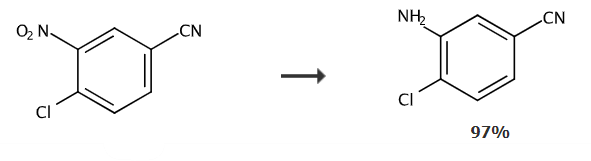
To a suspension of 4-chloro-3-nitrobenzonitrile (0.01 mol) in a mixture of EtOH (10 mL) and H2O (0.5 mL), iron powder ( 0.03mol) and CaCl2 (0.01mol). The resulting suspension was stirred at 60°C for 30 minutes. The reaction progress was monitored by TLC. Upon completion, the reaction mixture was filtered to remove iron residue, which was washed with EtOAc (2 × 20 mL). The organic extract was washed with H2O (3×10mL), brine (2×10mL), and dried over Na2SO4. The organic phase was evaporated and the residue was loaded directly onto a silica column and eluted with PE-EtOAc (20% EtOAc/Hexane) to give 3-amino-4-chlorobenzonitrile as an off-white solid, Yield 90%. mp 86-89°C, 1H NMR (400 MHz, DMSO-d6): δ = 7.66 (dd, J = 8.1, 7.5 Hz, 2 H), 7.56 (d, J = 7.6 , 7.3 Hz, 1 H), 4.25 (br s, 2 H). 13C NMR (100.6 MHz, DMSO-d6): δ = 146.2, 136.1, 131.1, 130.3, 129.2, 129.1, 128.2. IR (KBr): νmax=3395, 3050, 2953, 2229, 1546, 1325, 1269, 775cm-1. MS (EI): m/z=152.02.
Main reference materials
[1] [Chinese invention] CN200910054845.8 4-(4-methoxybenzylamino)-3-(5-nitro-4-thiocyanatopyrimidin-2-ylamino)benzonitrile and Its preparation method and use
[2] Chandrappa S , Vinaya K , Ramakrishnappa T , et al. An Efficient Method for Aryl Nitro Reduction and Cleavage of Azo Compounds Using Iron Powder/Calcium Chloride[J]. Synlett, 2010, 2010(20):3019 -3022.

 微信扫一扫打赏
微信扫一扫打赏

