Background and overview[1]
(4S,5R)-2-(4-Methoxyphenyl)-4-phenyl-3,5-oxazolidinedicarboxylic acid 3-tert-butyl ester is a pharmaceutical intermediate, which can be made from ( 2′R,3′S)-phenylisoserine methyl ester and p-toluic acid were prepared in three steps. There are reports in the literature that (4S,5R)-2-(4-methoxyphenyl)-4-phenyl-3,5-oxazolidinedicarboxylic acid 3-tert-butyl ester can be used to prepare an anti-tumor activity A novel taxane antitumor compound derived from the 2′-derivatized side chain of docetaxel.
Preparation[1]
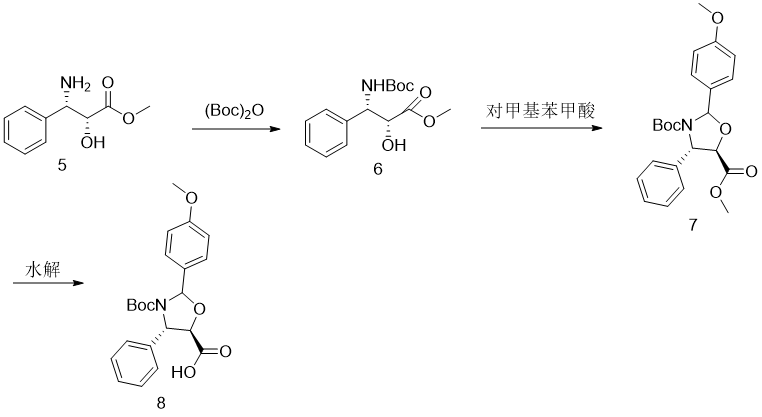
Step 1. Synthesis of (2′R,3′S)-N-Boc-phenylisoserine methyl ester 6
Add 50mmol of (Boc)2O dissolved in CH2Cl2 with CH2Cl 2/H2O dissolved (2′R,3′S)-phenylisoserine methyl ester 50mmol and 3.58g potassium hydroxide solution, react at room temperature Carry out, TLC monitors that after the reaction is complete, add H2O to the reaction solution, let it stand for layering, separate the organic phase, and use CH2Cl2 for the aqueous phase. Extract, combine the organic phases, wash with water, and dry with anhydrous MgSO4. The solvent was removed to obtain a crude product, which was recrystallized to obtain (2′R,3′S)-N-Boc-phenylisoserine methyl ester with a yield of 93.2%.
Step 2, synthesis of phenyloxazoline carboxylic acid methyl ester compound 7
Dissolve 46.4 mmol of compound 6, 51 mmol of p-methoxybenzaldehyde and 0.1 g of pyridinium p-toluenesulfonate in toluene and reflux. TLC monitors that the reaction is complete and then cools to room temperature and removes the solvent to obtain compound 7.
Step 3. Synthesis of (4S,5R)-2-(4-methoxyphenyl)-4-phenyl-3,5-oxazolidinedicarboxylic acid 3-tert-butyl ester 8
Dissolve the above product 7 with MeOH, add dilute NaOH, and stir at room temperature. After TLC monitors that the reaction is complete, MeOH is removed. The remaining residue is dissolved and diluted with AcOEt/H2O, and allowed to stand for separation. Add AcOEt to the aqueous phase and adjust the pH to acidic with dilute hydrochloric acid. The mixture was allowed to stand and separated into layers, and the organic phase was separated. The aqueous phase was extracted with AcOEt. The organic phases were combined, washed with water, and dried with anhydrous MgSO4. The solvent was removed to obtain phenyloxazoline carboxylic acid 8. , the total yield of the two steps is 92%, m.p.132-133℃. 1HNMR(400MHz, CDCl3)δ7.50-7.31(m,7H),6.94(d,J=8.6Hz,2H),6.41(s,1H ),5.42(s,1H),4.64(d,J=4.2Hz,1H),3.83(s,3H).13CNMR(100MHz,CDCl3)δ172.73,160.44,151.71,128.89,128.33,128.17,126.32,114.00, 92.44,82.57,81.14,63.68,55.32,27.82.
Main reference materials
[1][Chinese invention] CN201811188548.8 A docetaxel-1,2,3,-triazole compound and its synthesis process and application

 微信扫一扫打赏
微信扫一扫打赏

