Background and overview[1]
(2S-cis)-(+)-2,3-dihydro-3-hydroxy-2-(4-methoxyphenyl)-1,5-benzothiazepine-4(5H) -Ketone is a key intermediate in the synthesis of diltiazem. The current route to synthesize diltiazem is mainly to first synthesize (2S-cis)-(+)-2,3-dihydro-3-hydroxy-2-(4-methoxyphenyl)-1,5-benzothiazepine Zo-4(5H)-one is then obtained through a two-step reaction of N-alkylation and O-acetylation. The chemical name of diltiazem is cis-(+)-5-[(2-dimethylamino)ethyl]-2-(4-methoxyphenyl)-3-acetoxy-2,3- Dihydro-1,5-benzothiazepine-4-(5H)-one hydrochloride is a selective calcium channel blocker. Diltiazem is a benzodiazepine calcium antagonist. It was originally developed by the Japanese company Tanabe and was first launched in Japan in the early 1970s. Later, it was successively sold in the United States and Japan under the names of diltiazem, diltiazem, and siltiazem. , France, Spain and other countries.
Preparation[1]
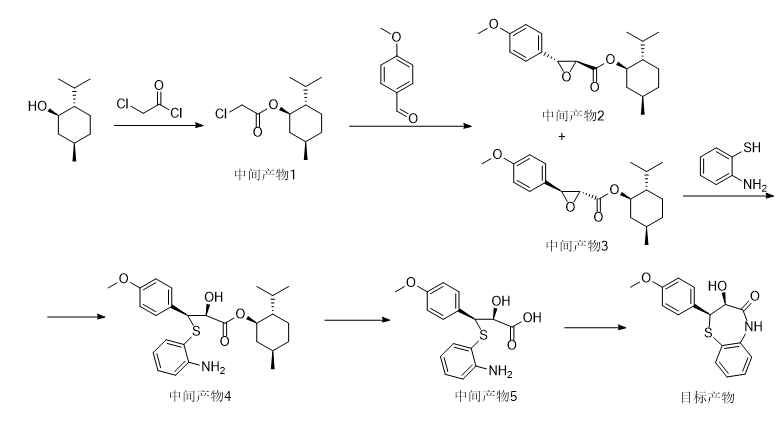
Add 62.5g of L-menthol, 0.5g of N,N-dimethylaminopyridine (DMAP), and 200mL of methylene chloride into a 1L reaction bottle, and stir to dissolve. Add 63.0g of sodium carbonate, add 56.5g of chloroacetyl chloride dropwise at a controlled temperature of 25°C to 30°C, and keep stirring at 25°C to 30°C for 2 hours after dripping. After the reaction, 400 mL of water was added, and after stirring and liquid separation, the organic layer was dried by adding 10 g of anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain intermediate product 1, which was directly used in the next reaction without purification.
Add 108g of sodium methoxide (30% methanol solution) and 100mL of methanol into a 1L reaction bottle, and cool down to -5℃~0℃. Add 49 g of anisaldehyde and 100 mL of methanol to the intermediate product 1 obtained in any one of the above embodiments 1 to 3, stir and dissolve evenly, then slowly drop it into the reaction bottle, control the temperature of the dropwise addition to -5°C to 0°C, and keep it at 0°C after the dripping. Stir the reaction at ~5°C for 2 hours. After the reaction is completed, the reaction solution is slowly poured into 700mL of ice water, stirred for 0.5h and then filtered. Add 400mL of ethyl acetate to the filter cake, stir and dissolve, then separate the aqueous layer, dry the organic layer with 10g of anhydrous sodium sulfate and filter, and stir in the filtrate. Slowly add 800 mL of n-heptane, a large amount of solid precipitates, filter, and dry to obtain intermediate product 3, with a yield of 66.2 g, a yield (based on anisaldehyde) of 55.3%, and an optical purity of 99.1%.
Add 66.2g of the intermediate product 3 obtained in any one of the above Examples 4 to 6 and 300mL of toluene into a 500mL reaction bottle, and raise the temperature to 105°C. After reaching the temperature, 26.0g of o-aminothiophenol is quickly added dropwise, and the dripping is completed at 110°C. Reflux reaction at ~112°C for 4 hours. After the reaction, the reaction solution was cooled to below 5°C, filtered, and the filter cake was washed with a small amount of cold toluene and dried to obtain intermediate product 4, with a yield of 71.3g and a yield (based on intermediate product 3) of 78.2%.
Add 71.3g of the intermediate product 4 obtained in any one of the above Examples 7 to 9 into a 500mL reaction bottle, add 200mL of 5% sodium hydroxide aqueous solution, raise the temperature to 40°C and stir for 2 hours. After the reaction is completed, adjust with 6mol/L hydrochloric acid. When the pH reaches 3.0-3.5, a large amount of solid is precipitated and filtered. After the filter cake is washed with water, the wet product 5 of the intermediate product is directly used in the next step of the reaction.
Add the wet intermediate product 5 and 500 mL of toluene into a 1L reaction bottle, heat to 50°C to dissolve, separate the lower aqueous layer, add 10 μL of methanesulfonic acid to the organic layer, heat to 110°C ~ 112°C, reflux and separate water for 8 hours . After the reaction, the reaction solution was cooled to 0°C and kept for crystallization for 4 hours. It was filtered. The filter cake was washed with a small amount of ice and toluene and then scraped out and dried to obtain 40.5g of yellow solid product with a purity of 99.2%. The total yield of the two steps of hydrolysis and cyclization was 86.3%.
Main reference materials
[1] [China invention, China invention authorization] CN201511010934.4 Selective synthesis method of diltiazem chiral intermediate

 微信扫一扫打赏
微信扫一扫打赏

