Background and overview[1]
2-Iodoaniline belongs to aniline. Aniline is an important organic synthon that can be used in the synthesis of phenol, benzonitrile and other compounds. It is also an important organic chemical raw material and chemical intermediate, and chemical products prepared from it There are more than 300 kinds, which are widely used in industries such as dyes, medicines, pesticides, explosives, spices, rubber vulcanization accelerators, etc. It is an important raw material for the production of polyurethane, MID (diphenylmethane diisocyanate), and there is currently an annual global demand for it. The volume exceeds 5 million tons, and the development and utilization prospects are very broad.
Preparation[1-2]
Report 1,
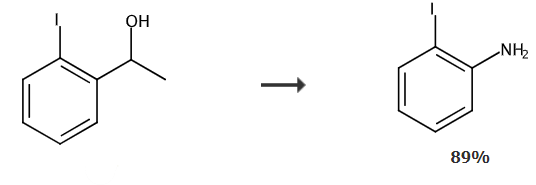
Take a reaction tube, add 50 mg of sodium azide, 75 mg of 1-(2-iodophenyl)ethanol, 300 uL of trifluoroacetic acid, 150 uL of methylsulfonic acid, and 1.0 mL of n-hexane, and stir at 40°C for 24 hours. After the reaction, 10 mL of sodium hydroxide solution was added to quench the reaction, extracted three times with ethyl acetate, and the organic phase was washed with 5 mL of brine. The organic phases were combined and separated by column chromatography to obtain 58.4 mg of o-iodoaniline with a yield of 89%. 1H NMR (CDCl3, 400MHz): δ7.66 (d, J=7.9Hz, 1H), 7.16 (t, J=7.6Hz, 1H), 6.77(d,J=8.0Hz,1H),6.50(t,J=7.5Hz,1H),4.09(s,2H);13C NMR (CDCl3, 100MHz): δ146.69, 138.94, 129.29, 119.92, 114.68, 84.13ppm; MS (70eV): m/z (%) = 219.0.
Report 2,

Add 0.5mmol aniline to 4mL of water, add 0.125mmol copper acetate, 1.0mmol potassium iodide, and 1.0mmol hydrogen peroxide, and react at room temperature for 2 hours. After the reaction is completed, add saturated NaCl aqueous solution to the reaction solution, and use acetic acid to Extract with ethyl ester, dry the organic layer over anhydrous magnesium sulfate, filter, and evaporate to dryness under reduced pressure at 60°C to obtain crude 2-iodoaniline. Put the crude 2-iodoaniline into silica gel column chromatography, use a solution with a volume ratio of ethyl acetate and petroleum ether of 1:5 as the mobile phase, track and collect the eluent with an Rf value of 0.3-0.5 by TLC, and collect the obtained eluate. Deliquify, remove the solvent under reduced pressure, and dry to obtain 40 mg of pure 2-iodoaniline with a yield of 37%. 1H NMR (500MHz, CDCl3) δ7.65 (dd, J=7.8, 1.2Hz, 1H), 6.83 (td, J=7.6, 1.3Hz, 1H), 6.79 (td, J=7.7, 1.3Hz, 1H), 6.31 (dd, J=7.8, 1.3Hz, 1H), 4.01 (s, 2H). 13C NMR (126MHz, CDCl3) δ 148.3, 138.4, 128.4 ,120.3,114.4,83.9.
References
[1] [Chinese invention] CN201811064304.9 A synthesis method of aromatic amine compounds
[2] [Chinese invention, Chinese invention authorization] CN201611085542.9 A method for preparing ortho-halogenated aromatic amines based on C-H activated aromatic amines [Public]/A method for preparing ortho-halogenated aromatic amines based on C-H activated aromatic amines Method【Authorization】

 微信扫一扫打赏
微信扫一扫打赏

