Background and overview[1]
2-Nitro-5-chloroaniline is an important pharmaceutical and veterinary drug intermediate. It is currently used in fenbendazole, an upgraded product of the veterinary drug albendazole, and has broad market prospects.
Preparation[1]
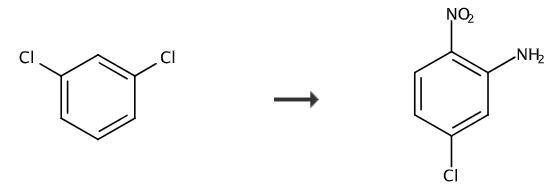
Synthesis of 1, 2,4-dichloronitrobenzene
Add 0.714mol concentrated sulfuric acid into a 250mL flask, then slowly add 0.701mol 95% nitric acid dropwise, cool and set aside. Add 0.68 mol m-dichlorobenzene to a 250 mL three-necked flask, lower the temperature in an ice bath to below 20°C, slowly add the above mixed acid dropwise, pay attention to control the reaction temperature between 35 and 45°C, and continue to react at 45°C for 1 hour after the dropwise addition. . The acid layer is separated after standing, and the obtained product is washed with water and alkali to obtain crude 2,4-dichloronitrobenzene. The crude product was added to 200 mL of 95% ethanol and heated to dissolve, then stirred and cooled to 10°C. A large number of needle-like crystals were formed. After filtration and drying, 105 g of product was obtained. The mother liquor was concentrated and continued to crystallize to obtain 14 g of product. The total yield was 91.1%. The purity measured by chromatography is 99.2%.
Synthesis of 2, 2-nitro-5-chloroaniline
Add 2.46mol 2,4-dichloronitrobenzene and 7.72mol toluene into the 3L autoclave, seal the autoclave, replace the air with nitrogen, then introduce 14.1mol liquid ammonia, raise the temperature to 160°C and continue the reaction for 8 hours. Then cool the temperature to 40°C, drain off the excess ammonia, open the kettle, add the solid-liquid mixture obtained to 800mL of water, continue to cool to 10°C, filter, add the obtained filter cake to the water, continue to beat and filter, and the obtained solid is crystallized with methanol 388g of pure product was obtained, with a yield of 91.2%, a liquid phase external standard purity of 99.5%, and a melting point of 126~129°C.
Apply[2-3]
Application 1,
CN201811013064.X reports a new method for the preparation of albendazole. Albendazole, also known as albendazole, chemical name 5-propylthio-1H-benzimidazole-2-carbamate methyl ester, is a broad-spectrum, highly efficient and low-toxic anthelmintic drug produced by SmithKline in the United States. The company first went on the market in 1977. Currently, the drug is widely used in the clinical treatment of various helminth infectious diseases. It has a strong repellent effect on nematodes, flukes, and tapeworms parasitic in humans and animals. It is also effective against human cysticercosis and intestinal nematodes. The effective rate is as high as 100%, and it can significantly inhibit the development of insect eggs and has no accumulation effect in the body. The invention provides a brand-new synthesis route of albendazole, which uses 2-nitro-5-chloroaniline as the starting material and obtains high-purity albendazole through substitution, condensation, reduction and cyclization reactions. It is characterized in that 2-nitro-5-chloroaniline undergoes a substitution reaction and is then condensed with halogenated n-propane to obtain 2-nitro-5-propylthioaniline, thereby avoiding the use of propylamine which is expensive and has an irritating odor. Thiols. Avoid using the high-risk hydrogenation reduction process, adopt the alkali sulfide reduction process with cheap and easily available raw materials, and provide a set of non-toxic treatment solutions for reducing stratified wastewater. At the same time, the by-product sodium thiosulfate can be obtained, turning waste into treasure. The new process has high yield, low safety risk, and is easy for industrial production. The prepared albendazole has high content and small individual impurity content, and complies with the latest standards such as the United States Pharmacopeia and the European Pharmacopoeia.
Application 2,
CN201610912785.9 discloses a synthesis method of 1,4-diiodo-2-methoxy-5-nitrobenzene. In the process of DNA sequencing technology, the breakable connection unit connects nucleotides and fluorescein, and its properties directly determine key indicators such as sequencing efficiency and read length. 1,4-diiodo-2-methoxy-5-nitrobenzene compound is an important intermediate for this cleavable linking unit. The synthesis steps include: (1) 2-nitro-5-chloro-aniline reacts with N-iodosuccinimide to generate 2-nitro-4-iodo-5-chloroaniline; (2) 2-nitro -4-iodo-5-chloroaniline reacts with alkali to obtain 3-methoxy-4-iodo-5-nitro-aniline; (3) 3-methoxy-4-iodo-5-nitro-aniline and After mixing p-toluenesulfonic acid, the nitrite solution is added dropwise and the reaction is stirred, and then the reaction liquid is added dropwise to the KI solution to react to generate 1,4-diiodo-2-methoxy-5-nitrobenzene. The synthesis method of the present invention has easy-to-obtain raw materials, mild reaction conditions, high selectivity, good post-processing operability, high yield, easy scale-up of production, and is more environmentally friendly.
References
[1]Xu Guojun. Research on the synthesis of 5-chloro-2-nitroaniline[J]. Fine and Specialty Chemicals, 2012, 20(11):45-46.
[2][Chinese invention] CN201811013064.X A new method for the preparation of albendazole
[3][Chinese invention] CN201610912785.9 A synthesis method of 1,4-diiodo-2-methoxy-5-nitrobenzene

 微信扫一扫打赏
微信扫一扫打赏

