Background and overview[1]
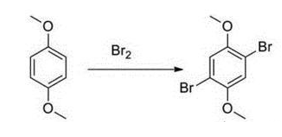
1,4-dibromo-2,5-dimethoxybenzene is an organic intermediate that can be used to prepare 1,4-dibromohydroquinone from hydroquinone dimethyl ether and elemental Bromine reaction preparation.
Preparation[1-2]
Method 1:
Synthesis of 1,4-dibromo-2,5-dimethoxybenzene: Add 20.0g of hydroquinone dimethyl ether (0.145mol, 1.0eq), 55ml of glacial acetic acid into a 250ml reaction bottle, and conduct ultrasound. Dissolve; add dropwise a solution of 15ml Br2 (0.290mol, 2.0eq) and 15ml glacial acetic acid at room temperature, complete the dripping after 1.5 hours, stir at room temperature for 2.5h; place in the upper shelf of the refrigerator and cool to below 10°C (Do not solidify the acetic acid) Suction filtration; and wash the filter cake with petroleum ether to obtain a white solid, which weighs 35.6g after vacuum drying, with a yield of 83.6%. 1HNMR (400MHz, CDCl3) δ7.11 (s, 2H), 3.85 (s, 6H).
Method 2:
Add 1.5ml acetonitrile, 0.5mmol 1,4-dimethoxybenzene, 0.65mmol liquid bromine (Br2) and 0.041mmol nitrogen dioxide into a reaction tube of about 45ml. Then add the magnet, seal the reaction tube, place the reaction tube into a reaction tank with a temperature of 30°C, and stir magnetically for 24 hours. After the reaction, the reaction system was cooled to room temperature, and the internal standard 1,2,4,5-tetramethylbenzene was added. Gas chromatography analysis showed that the internal standard yield of the product was 76%. Repeat the above experimental process, but without adding internal standard substance, use column chromatography to separate and purify the obtained product, and obtain the 1,4-dibromo-2,5-dimethoxybenzene product.
Apply[1]
1,4-Dibromo-2,5-dimethoxybenzene can be used to synthesize 1,4-dibromohydroquinone: In a 100ml single-neck bottle, add 1,4-dibromo-2,5 -Dimethoxybenzene 7.1g (24.2mmol, 1.0eq) and 30ml methylene chloride were dissolved. Add BBr34.6ml (49.6mmol, 2.05eq) and reflux for 24 hours. Evaporate off the methylene chloride, add 50 ml of water while stirring, filter with suction and wash the filter cake with water. After vacuum drying, 5.08 g of white solid was obtained, with a yield of 79%. 1HNMR (400MHz, CDCl3) δ7.16 (s, 2H), 5.14 (s, 2H).
References
[1]CN201610811308.3 Convenient synthesis method of aromatic diboron esters suitable for large-scale production
[2]CN201410511647.0 Preparation method of dibromobenzene compounds with alkoxy groups

 微信扫一扫打赏
微信扫一扫打赏

