Background and overview[2]
3-Fluorophenylacetylene can be used as a pharmaceutical synthesis intermediate and is a terminal alkyne. The addition reaction of terminal alkynes is widely used in the synthesis of drugs, pesticides and organic functional materials. The reason why this type of reaction is important is because there is an alkyne bond in the product, which can be further functionalized, so it is an important type of organic Synthetic intermediates.
Apply[1-2]
Report 1,
3-Fluorophenylacetylene can be used to prepare 3-fluoroethane: combine the catalyst [(IPr)AuCl] (3.1mg, 0.5mol%), 3-fluorophenylacetylene (1mmol), methanol (1ml) and water ( 0.5ml) was added to the 25ml reactor in sequence. The reaction mixture was reacted at 110°C for 6 hours and then cooled to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained through column chromatography (developing solvent: petroleum ether/ethyl acetate), yield: 97%.1HNMR (500MHz, CDCl 3) δ7.74 (d, J=7.10Hz, 1H, ArH), 7.63 (d, J=8.90Hz, 1H, ArH), 7.45 (q, J=7.10Hz, 1H, ArH), 7.30-7.24 (m, 1H, ArH), 2.60 (d, J=0.85Hz, 3H, CH3); 13CNMR (125MHz, CDCl3) δ196.47 , 162.62 (d, JC-F=245.65Hz), 138.99 (d, JC-F=5.8Hz), 130.55 (d, JC-F=7.47Hz), 123.94, 119.84 (d, JC-F=22.00Hz) , 114.63(d, JC-F=22.63Hz), 26.35
Report 2,
The addition reaction of trifluoromethyl ketone and terminal alkynes is widely used in the synthesis of drugs, pesticides and organic functional materials. There are two reasons why this type of reaction is important: (1) There is an alkyne bond in the product , can be further functionalized, and is therefore an important type of organic synthesis intermediate; (2) while forming a carbon-carbon, a CF3 group is introduced into the target molecule, which is a potentially biologically active and special A group of physical properties, because 30% of currently synthesized drugs and 20% of pesticides contain fluorine atoms. The synthesis example is as follows:
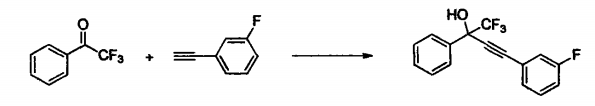
Add 2,2,2-trifluoroacetophenone (0.5mmol), 3-fluorophenylacetylene (1.0mmol), CuOH (0.05mmol), K2CO3 (0.1mmol) and DMA (1mL) to the Xulinker bottle , stir the reaction at 50°C for 24 hours, and follow the reaction with thin layer chromatography. After the reaction, 15 mL of saturated brine was added to quench the reaction. The reaction mixture was extracted with dichloromethane (15 mL × 3). The organic phases were combined and concentrated using a rotary evaporator to obtain the crude product. The target product was obtained by column chromatography. The eluent used for analysis was petroleum ether:ethyl acetate (10:1), and the product structure was identified by NMR and high-resolution mass spectrometry. The isolation yield reached 68%.
References
[1]CN201510103316.8 A method for synthesizing ketones by hydrolyzing alkynes
[2]CN201410325953.5 A simple and efficient method for preparing efavirenz intermediates

 微信扫一扫打赏
微信扫一扫打赏

