Background and overview[1][2]
Dibenzofuran-based optoelectronic materials are widely used in blue organic light-emitting devices, and their development and research value are very high. As an important intermediate, 1-bromodibenzofuran is used in the synthesis of existing technologies. The overall yield of 1-bromodibenzofuran is low and the process route is long, resulting in higher costs. Waste gas and waste water are generated during the reaction, which is highly polluting and dangerous. It also requires high equipment and complex post-processing, resulting in a long production cycle. .
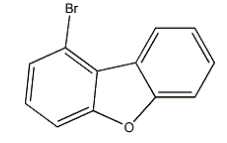
1-Bromodibenzofuran
Apply[3]
1-Bromodibenzofuran is one of the separated products of coal tar. It has large output, wide range of uses and low price. Its derivatives are one of the important fine chemicals and are used in medicine, pesticides, dyes and organic luminescence. materials and other fields. 1-Bromodibenzofuran is one of the derivatives of dibenzofuran. It is widely used in the synthesis of organic light-emitting materials. It is an important material intermediate and the study of its synthesis method is of great significance.
Preparation [2]
Regular Line 1
The total yield of conventional line 1 is 38.4%, and the cost is about 12,000. Boron tribromide is used in the third step of the process. The hydrogen bromide gas generated during the post-treatment process of this material is extremely large and releases emissions. The heat is intense, and a lot of acidic water is produced, which causes a large loss of equipment and poses a great safety hazard. The post-processing of this process is more complicated, resulting in a longer time. Taking the production of 3Kg as an example, this process takes 20 days.
Regular Line 2
The total yield of conventional route 2 is 41%, and the cost is about 18,000. The obtained product has a heavy pigment and is difficult to purify to 99% purity. The process has five steps. Taking 3Kg as an example, the cycle is 23 days. And it involves diazonium salt reaction, so the operation is dangerous. The methods currently reported in the literature have complex processes and large post-processing pollution, so it is of great significance to develop simple and green synthesis methods.
Specific method:
Step 1) Use m-fluorobenzene, trimethyl borate and lithium diisopropylamide to react at low temperature to generate 2-bromo-6 fluorobenzene boric acid, in which m-fluorobenzene, trimethyl borate and diisopropylamide The molar ratio of lithium amide is 1~1.5:1~2:1~1.5;
Step 2) Utilize the 2-bromo-6-fluorophenylboronic acid and potassium difluoroborate generated in step 1) to undergo a salt-forming reaction to generate 2-bromo-6-fluoro-phenyl potassium trifluoroborate, in which 2-bromo- The molar ratio of 6-fluorobenzene boric acid and potassium difluoride is 1:3~5;
Step 3) The coupling reaction between the 2-bromo-6-fluoro-phenyl potassium trifluoroborate generated in step 2) and o-bromophenol generates 2′-bromo-6′-fluorobiphenyl-2- Alcohol, in which the molar ratio of 2-bromo-6-fluoro-phenyl potassium trifluoroborate to o-bromophenol is 1:1~1.3;
Step 4) The 2′-bromo-6′-fluorobiphenyl-2-ol generated in step 3) undergoes a ring closing reaction to generate 1-bromodibenzofuran.
Preferably, the specific reaction steps of step 1) are: in a nitrogen-protected environment, add tetrahydrofuran to the reaction kettle, then add lithium diisopropylamide, start stirring and cool down to -78°C, and then drop Add the tetrahydrofuran solution of m-fluorobromobenzene. After the dropwise addition is complete, a large amount of solid will precipitate. Keep it at -78°C for 1 to 2 hours. Then add trimethyl borate dropwise. After the dropwise addition, the system will become clear. Let it naturally rise to room temperature to complete the reaction. Then At 50°C, evaporate the tetrahydrofuran under reduced pressure, then pour the remaining reaction solution into the prepared dilute hydrochloric acid aqueous solution, stir for 1 hour, a large amount of white solid will precipitate, filter it with suction, obtain a filter cake, and then use petroleum ether to Heat beating at 50°C for 0.5h, then lowered to room temperature, and filtered with suction to obtain 2-bromo-6fluorophenylboronic acid.
Preferably, the molar ratio of m-fluorobenzene, trimethylborate and lithium diisopropylamide is 1:1.2:1.
Main reference materials
[1] Yu Jing, Zhang Xingchuan, Wang Zunyao, & Zeng Xiaolan. (2006). Density functional theory study on the thermodynamic properties and stability of polybrominated dibenzofurans. Acta Chimica Sinica, 64(19), 1961- 1968.
[2] Du Xihua. Quantitative structure-activity relationship of thermodynamic properties of polybrominated dibenzofurans/thiophenes. Journal of Chemical Engineering, 61(12).
[3] Miao Zongcheng, Ji Jing, Liu Cong, & Zhao Yang. (2016). Preparation of new organic electroluminescent material intermediate b-2-dibenzofuranylboronic acid. Applied Chemical Engineering, v. 45;No.287(01), 80-82+86.

 微信扫一扫打赏
微信扫一扫打赏

