Background and overview[1]
3-Bromo-2-fluorobenzonitrile is an organic intermediate that can be prepared from 3-bromo-2-fluorobenzoic acid in two steps. 3-Bromo-2-fluorobenzonitrile can be used as a pharmaceutical intermediate for the preparation of STK4 inhibitors.
Preparation[1]
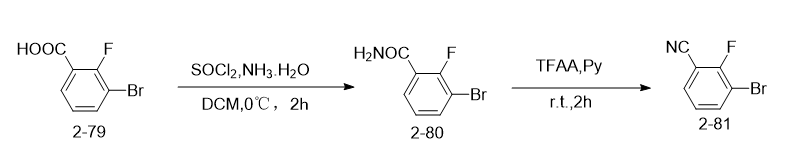
Step 1, preparation of 3-bromo-2-fluorobenzamide (2-80)
3-Bromo-2-fluorobenzoic acid (2-79, 400 mg, 1.83 mmol) and SOCl2 (4.39 mg, 3.66 mmol) were dissolved in DCM (5 mL) at room temperature. The mixture was stirred for 1 h, and then ammonia (1 mL) was added. The resulting mixture was then stirred at room temperature for 2 h. After the reaction was completed, the resulting mixture was washed with water and extracted with ethyl acetate (150 mL × 3). The combined organic phases were concentrated under reduced pressure, and the resulting residue was purified by silica gel chromatography (petroleum ether/ethyl acetate = 1/1) to obtain intermediate 2-80 (white solid, 300 mg, 75% yield ). LCMS (m/z): 218 [M+H]+.
Step 2, preparation of 3-bromo-2-fluorobenzonitrile (2-81)
3-Bromo-2-fluorobenzamide (2-80, 260 mg, 1.2 mmol), TFAA (471 mg, 2.4 mmol) and pyridine (Py) (474 mg, 6.0 mmol) were dissolved in diox The mixture in alkane (5 mL) was stirred at room temperature for 4 h. After the reaction was completed, the resulting mixture was washed with water and extracted with ethyl acetate (150 mL × 3). The combined organic phases were concentrated under reduced pressure, and the resulting residue was purified by silica gel chromatography (petroleum ether/ethyl acetate = 1/1) to obtain intermediate 2-81 (white solid, 100 mg, 42% yield).
Apply[1]
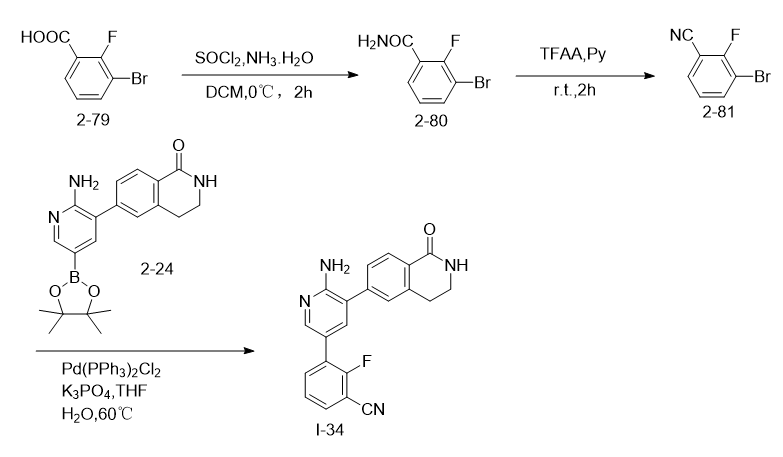
3-Bromo-2-fluorobenzonitrile can be used to prepare 3-(6-amino-5-(1-oxo-1,2,3,4-tetrahydroisoquinolin-6-yl)pyridine- 3-yl)-2-fluorobenzonitrile (I-34). I-34 is an inhibitor of STK4 (also known as MST1), a serine-threonine kinase that is part of the Hippo signaling pathway. STK4 is involved in multiple cellular processes, including proliferation, transport, apoptosis, immune response, and stress response.
References
[1] From PCT Int. Appl., 2016161145, 06 Oct 2016

 微信扫一扫打赏
微信扫一扫打赏

