Background and overview[1-2]
4-Aminomethylbenzoic acid methyl ester hydrochloride is a pharmaceutical intermediate, which can be prepared from 4-aminomethylbenzoic acid through one-step esterification, or from 4-(bromomethyl)benzoic acid methyl ester Prepared in two steps.
Preparation[1-2]
Report 1,
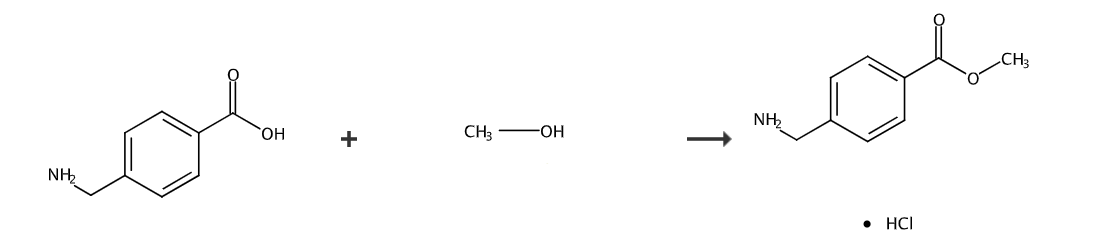
Dissolve 10g (151g/mol, 0.066mol) 4-aminomethylbenzoic acid in 150ml methanol, and slowly add 7.15ml (118g/mol, 1.64g/ml, 0.099mol) into the solution under an ice-salt bath. ) Thionyl chloride, keep the temperature below 10°C, complete the dropwise addition, continue to stir the reaction for 1 hour, then heat to 70°C and reflux for 5 hours. Thin layer chromatography shows that the reaction is complete. Recover methanol and excess thionyl chloride under reduced pressure. , the residue was washed with ethyl acetate/petroleum ether 1:3 to obtain 9.7g of bright white solid. The melting point of the product is 182-183°C.
Report 2,

A) 4-(azidomethyl)-benzoic acid methyl ester
Within 5 minutes, add 8.13g sodium azide to a solution of 5.73g 4-(bromomethyl)benzoic acid methyl ester in 30mL DMSO, and place it under stirring and TA for 3h30min. The reaction mixture is extracted with ether, the organic phase is washed with water, saturated sodium chloride solution, dried over sodium sulfate and the solvent is evaporated in vacuo. 4.72 g of the desired product were obtained as a colorless oil.
B) Methyl 4-aminomethylbenzoate hydrochloride
Cool the solution of 4.71g of the compound obtained in the previous step in 30mLTHF to 4°C, add 6.57g triphenylphosphine in batches within 30min, and leave it under stirring for 6h while allowing the temperature to return to TA. Then add 0.68 mL of water and place under stirring and TA for 16 h. The reaction mixture was extracted with AcOEt, the organic phase was washed with water, saturated sodium chloride solution, dried over sodium sulfate and the solvent was evaporated in vacuo. The residue was absorbed with diethyl ether, the insoluble matter was filtered out, the filtrate was concentrated in vacuo, and the chloroform/methanol/NH4OH mixture (90/10/0.2; v/v /v) elution and the residue was purified by chromatography. The product obtained was reworked in methanol, 10 N HCl was added until pH = 1 and concentrated in vacuo. 4.25 g of the desired product were obtained.
References
[1] Shi Xiangfei, Ye Qingquan, Xue Yang, Zhao Yanjin, Li Shuxin. Synthesis and anti-tumor activity of benzamide histone deacetylase inhibitors [J]. Journal of Pharmaceutical Sciences of the People’s Liberation Army, 2011, 27(02): 99-102.
[2]FromPCTInt.Appl.,2002076964,03Oct2002

 微信扫一扫打赏
微信扫一扫打赏

