Background and overview[1]
2-Bromo-6-fluorophenol, also called 2-fluoro-6-bromophenol, can be used as a pharmaceutical synthesis intermediate. Using 2-fluorophenol as raw material, through sulfonation, halogenation and deprotection, the intermediate 2-fluoro-6-halogenated phenol can be obtained
Preparation[1]
Synthesis of 2-fluoro-6-bromophenol:
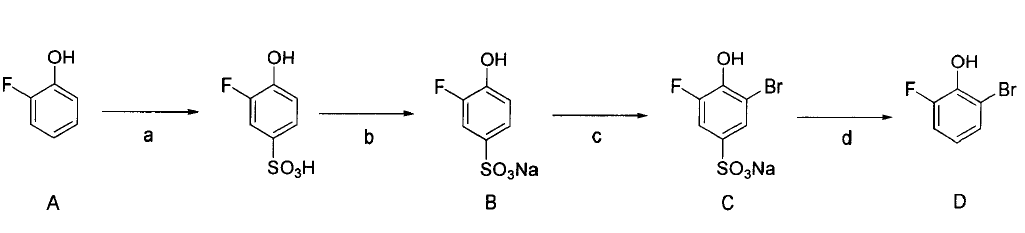
1) Synthesis of sodium 3-fluoro-4-hydroxybenzenesulfonate (B) (steps a and b, sulfonation reaction):
a: Add 112g (1.0mol) A to the reaction kettle, raise the temperature to about 120°C, and within 30 minutes, slowly add 80ml (1.5mol) 98% sulfuric acid dropwise while stirring. After the dropwise addition was completed, the reaction was continued at 120°C for 5 hours. After the reaction is completed, cool to below 100°C.
b: Add 400 ml of saturated sodium chloride solution to the above reaction mixture, and a large amount of sodium salt will precipitate. After complete cooling, filter and wash twice with 10% sodium chloride solution to obtain 192.6 g of product B with a yield of 90%.
2) Synthesis of sodium 3-fluoro-6-bromo-4-hydroxybenzenesulfonate (C) (step c, halogenation reaction):
c: Before the reaction, all The raw materials and equipment are treated anhydrously, and the bromine is washed twice with concentrated sulfuric acid and dehydrated. 250ml three-neck bottle equipped with mechanical stirrer, thermometer, separatory funnel and reflux condenser. Add 171.2g (0.8mol) B, 11.2g iron powder and 120ml carbon tetrachloride to the flask. Stir and heat to 55°C in a water bath, control the temperature at 50-60°C, and add 134.4g (0.84mol) bromine dropwise within 4 hours. After adding, keep warm and stir for 2 hours. After cooling, filter, and the filtrate is washed with water, alkaline water, and water in sequence. After drying, the solvent is evaporated to obtain 199 g of product C with a yield of 85%.
3) Synthesis of 2-fluoro-6-bromophenol (D) (step d, deprotection reaction):
d: Add 146.5g (0.5 mol)C and 200ml 70% sulfuric acid solution. After reacting at 180°C for 5 hours, it was cooled to room temperature, and 200 ml of methylene chloride was added for extraction. The extract was washed, dried, and the solvent was evaporated to obtain 76.4 g of oily product D, with a yield of 80%.
References
[1]CN200510008983.4 A preparation of 1,2-dialkoxy-3-fluorobenzene via the intermediate 2-fluoro-6-halogenated phenol method

 微信扫一扫打赏
微信扫一扫打赏

