Background and overview[1]
Benzoyl chloride is a colorless, transparent, irritating, tear-inducing liquid. The relative density is 1.215~1.284, the melting point is -6℃, and the boiling point is 197℃. It undergoes hydrolysis and alcoholysis when encountering water and alcohol. It is easily soluble in ether and carbon disulfide. It is produced by hydrolysis of trichlorotoluene or by reacting with benzoic acid. There are many synthesis processes for benzoyl chloride. Benzoic acid and silicon tetrachloride can synthesize benzoyl chloride in benzene solution. This process has a low yield and complicated purification steps, and is only suitable for laboratory development; the synthesis process of benzoic acid and thionyl chloride does not generate benzoyl chloride. In addition, gaseous sulfur dioxide and hydrogen chloride are generated without any solid by-product residue, so the product is easy to separate and purify. However, the cost of the raw material thionyl chloride is relatively high. Although it has been researched and developed for a long time, it is still used in laboratory preparations; benzoic acid and light The gas reaction process is suitable for industrial production, has a high yield, and is relatively simple to purify. However, phosgene is highly toxic and causes environmental pollution. As people have higher and higher requirements for environmental quality, this process is gradually being eliminated. In view of the resource advantage of benzoic acid in our chemical fiber factory, we have carried out research on the synthesis of benzoyl chloride using benzoic acid and trichlorotoluene as raw materials. The reaction temperature, raw material ratio, catalyst selection and dosage and other process conditions were investigated, and qualified benzoyl chloride samples were prepared in the laboratory.
Chemical properties[2]
Benzoyl chloride is more stable than aliphatic acid chloride, but because it contains more active chlorine, it is highly chemically active and is mainly used as a benzoyl chloride.
⑴ Hydrolysis: Benzoyl chloride is easily hydrolyzed with boiling water and alkalis, and slowly with cold water. The hydrolysis product is benzoic acid or benzoic anhydride. This reaction is commonly used to prepare p-chlorobenzene-free High purity benzoic acid without formic acid impurities.
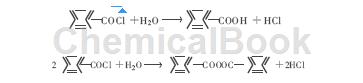
⑵ Reduction reaction: benzoyl chloride is hydrogenated and reduced by adding palladium catalyst to generate benzaldehyde:
![]()
⑶ Amination reaction: benzoyl chloride reacts with amines containing active hydrogen, and the benzoyl group can replace the hydrogen (Schotten-Baumamn reaction)
![]()
⑷Esterification reaction: benzoyl chloride reacts with an alcohol containing active hydrogen, and the benzoyl group replaces the hydrogen to form an ester.
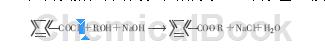
⑸ Condensation reaction: Benzoyl chloride is added to aluminum trichloride and reacts with equimolar aromatic hydrocarbons to produce a condensation reaction to generate benzophenone.
![]()
This is the Friedel-Crafts reaction, which is used to synthesize benzophenone and its series of derivatives in industry
⑹Peroxidation reaction: Benzoyl chloride interacts with sodium peroxide at 0°C to generate benzoyl peroxide.
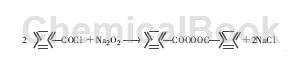
This is currently an important industrial use of benzoyl chloride.
⑺Chlorination reaction: Benzoyl chloride reacts with phosphorus pentachloride to form benzene trichloride.
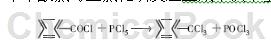
⑻ Salt formation reaction: benzoyl chloride reacts with sodium hydroxide to form sodium benzoate:
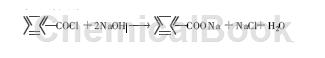
Apply[2]
Benzoyl chloride is mainly used in organic synthesis as a raw material for the preparation of organic peroxides, pesticides, Shilin dyes, benzotriazole ultraviolet absorbers, rubber antioxidants, medicines, etc. Benzoyl chloride is an important acylating agent and can be used to identify phenols, alcohols, amines, etc.
1 Benzoyl peroxide
Mainly used as an initiator for the polymerization of acrylic series resins, vinyl acetate resins, and MMA resins, or as a curing catalyst for polyester resin molding processing. It is also used as a bleaching agent for oil esters, waxes, and flour. Desiccant for unsaturated fats and fiber decolorizer.
2. Tert-butyl perbenzoate (TPB)
It is derived from the condensation of benzoyl chloride and tert-butyl peroxide. Mainly used as curing initiator for heat molding of unsaturated polyester and polymerization catalyst for resin.
3. 2,2′-dibenzamide diphenyl disulfide
It is derived from the reaction of bis(o-aminophenyl) disulfide and benzoyl chloride. Mainly used as peptizing agent for natural rubber and diene synthetic rubber.
4. Right, p’-dibenzoylquinonedioxime
It is derived from the reaction of p-quinonedioxime and benzoyl chloride. Mainly used as vulcanizing agent for butyl rubber, natural rubber and styrene-butadiene rubber, with good scorch resistance. It is especially suitable for butyl rubber to be used as inner tubes, water tires, vulcanization bladders and wires.
5. Dibenzoylthiamine
It is obtained by reacting vitamin B1 with benzoyl chloride in sodium hydroxide solution. Used as a fortifier for noodles, tofu and other foods.
6. Peroxybenzoic acid
Benzoyl peroxide is synthesized from benzoyl chloride and then combined withObtained from the oxidation of water. Peroxybenzoic acid is a strong oxidizing agent. Perbenzoic acid ethyl acetate synthesized with ethylene is used to analyze unsaturated compounds and determine the number of double bonds. Tert-butyl peroxybenzoate is used in silicone rubber and high-temperature vulcanized products.
7. Hippuric acid
It is prepared by synthesizing sodium benzoylaminoacetate from benzoyl chloride and aminoacetic acid in sodium hydroxide solution, and then acidifying it with hydrochloric acid. Mainly used as an intermediate for dispersing fluorescent yellow FFL and synthetic drugs.
8. 7-hydroxyflavone
It is obtained by benzoylation of 2,4-dihydroxyacetophenone and benzoyl chloride. Used as an intermediate for flavonoids as selective coronary artery dilators.
9. 2-Amino-5-chlorobenzophenone
Obtained from the catalytic reaction of p-chloroaniline and benzoyl chloride. It is a pharmaceutical intermediate used in the production of diazepam drugs, such as diazepam, sulfazepam, chlordiazepam, diazepam, nitrazepam, alprazolam, clonazepam, etc.
10. 2,5-Dichlorobenzoic acid
It is obtained by chlorination and hydrolysis of benzoyl chloride. Used in the synthesis of herbicides leguminosaur and dicaoping.
11. 2,5-Dichloro-p-phenylenediamine
After condensation of 2,5-dichloroaniline and benzoyl chloride, N-benzoyl-2,5-dichloro-4-nitroaniline is prepared by nitration, and then acidic hydrolysis generates 2,5-Dichloro-4-nitroaniline, obtained by reduction. It is used to synthesize azo dye CromophtalYellow6G and can also be used as a raw material for polyurethane synthesis.
12. Benzophenone
It is derived from the condensation of benzene and benzoyl chloride under the catalysis of aluminum trichloride. Benzophenone is an intermediate for ultraviolet absorbers, organic pigments, medicines, spices, and pesticides. Benzophenone can be used as heat carrier and calibration thermometer.
Preparation[1]
Benzoyl chloride synthesis process:
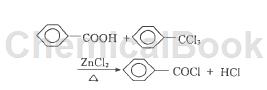
Add a certain amount of benzoic acid to a 500mL four-necked bottle, heat and melt it, then install a reflux condenser, thermometer, constant pressure dropping funnel and gas absorption device, and add a certain amount of benzoic acid to the four-necked bottle under stirring. Toluene trichloride and zinc chloride are heated and the temperature is controlled within a certain range. During the reaction, a large amount of hydrogen chloride is released and is absorbed by the water in the gas collecting bottle. The color of the reaction material in the reactor ranges from colorless to brown to yellow. Blue ※ Black, until no hydrogen chloride is released from the exhaust gas and the reaction stops. The obtained hydrogen chloride aqueous solution was diluted to various concentrations for utilization, and the obtained crude product was subjected to purification tests. Considering that the reactant trichlorotoluene and the product benzoyl chloride have high boiling points, in order to avoid high-temperature cracking during the product separation process, vacuum distillation is used to separate and purify the product in the laboratory. Put the crude product obtained by the reaction and the organic amine into a distillation bottle, install a packed glass vacuum distillation column and a condensation receiving system on the distillation bottle, perform vacuum distillation, and obtain colorless and transparent benzene of a certain purity. Acid chloride liquid.
Main reference materials
[1] Dictionary of Chemical Substances
[2] Synthesis and application of benzoyl chloride
[3] Research on the synthesis process of benzoyl chloride

 微信扫一扫打赏
微信扫一扫打赏

