Background and overview[1][2]
Cyclohexylphenylmethanone is an important pharmaceutical and dye intermediate, which is widely used in the synthesis of the antispasmodic drug cyclohexylphenylmethanol. In recent decades, there have been many reports on the research and production of such aromatic ketone compounds abroad, but domestically it started late and has few results. At present, most of the production of cyclohexyl phenyl ketone adopts the acylation method. This process route is divided into two steps. First, cyclohexylcarboxylic acid reacts with sulfoxide chloride to generate cyclohexyl acid chloride, and then cyclohexyl acid chloride reacts with benzene in trichloride. An acylation reaction occurs under the catalysis of aluminum chloride to generate cyclohexyl phenyl ketone. Both of these two-step reactions emit a large amount of acidic gas, and the reaction intermediate product cyclohexyl acid chloride and the catalyst aluminum trichloride are severely corrosive, the process consumes a lot of energy, and the production cost is high. Therefore, there is an urgent need to replace it with a production process that has low energy consumption and less environmental pollution. Many researchers at home and abroad have done a lot of work in this area and have made certain progress.
Apply[2-4]
Cyclohexylphenylmethanone is an important pharmaceutical and dye intermediate. It is mainly an important raw material for the synthesis of the antispasmodic drug cyclohexylphenylmethanol. It is also a photocuring agent for the synthesis of alpha-substituted products based on it. 1-Hydroxycyclohexylbenzophenone intermediate, its application examples are as follows:
1. Prepare a photoinitiator 184 (1-hydroxycyclohexyl phenyl ketone), including: high-temperature ketone production section: liquefy and mix benzoic acid and cyclohexylcarboxylic acid to prepare a reaction mixture, and mix the reaction After the liquid is preheated, it comes into contact with the metal salt catalyst, and is dehydrated and removed from carbon dioxide to produce ketone at high temperature (300-500°C) to obtain cyclohexyl phenyl ketone; one-pot chloride alkali hydrolysis section: cyclohexyl phenyl ketone, Using carbon tetrachloride and sodium hydroxide as reagents and tetrabutylammonium bromide as a phase transfer catalyst, a one-pot chlorination and alkaline hydrolysis reaction is performed to prepare 1?hydroxycyclohexyl phenyl ketone. The preparation process of the present invention is highly selective, safe and environmentally friendly.
2. Preparation method of S-α-cyclohexylbenzylamine. The specific method of the present invention is to use cyclohexylphenylmethanone as raw material, react with hydroxylamine to form oxime, and the oxime is hydrogenated and reduced to obtain racemic amine. The reaction product amine is combined with lipase, racemization catalyst, and acyl donor to perform a one-pot dynamic kinetic separation reaction. The reaction product is separated and then hydrolyzed to obtain S-α-cyclohexylbenzylamine. The invention has the characteristics of simple operation, good product yield, and high optical purity of the split product.
3. Preparation of oxaiodonium includes the following steps: S1. Preparation of 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxin: add cyclohexyl phenyl ketone, glycerol, Amberlyst-15 exchange resin and color-changing silica gel are mixed, heated, kept warm and stirred, filtered, the filtrate is washed with saturated sodium bicarbonate aqueous solution, left to stand, the organic layer is taken, dried, and distilled under reduced pressure to obtain material A; purify material A and dry to obtain 2-phenyl-2-cyclohexyl-4-methanol-1,3-dioxin; S2, preparation of 2-phenyl-2-cyclohexyl-4-piperidinylmethyl-1,3-dioxin; S3. Prepare oxaiodo ammonium. The invention has mild reaction conditions, simple operation, is suitable for industrial production, has low raw material toxicity, is cheap and easy to obtain, has low cost and good comprehensive yield.
Preparation[1,5]
Method 1: Reaction of cyclohexanecarbonyl chloride with benzene under the catalysis of AlCl3, the yield is 76%, the reaction equation is as follows:
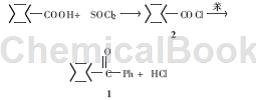
1) Cyclohexanecarbonyl chloride (2)
Add cyclohexanecarboxylic acid (32 g, 0. 25 mol) into the reaction bottle and heat to melt. Slowly add thionyl chloride (21 ml, 0.29 mol) dropwise at 50°C. The dropwise addition is completed in about 1.5 h. Heat and reflux until no more gas is released (about 2.5 h). The thionyl chloride was recovered under reduced pressure to obtain 2 (34 g, 92.8%).
2) Cyclohexyl phenyl ketone (1)
Add anhydrous aluminum trichloride (38 g, 0. 37 mol) and benzene (80 ml, 0. 90 mol) into the reaction bottle, slowly add 2 dropwise while stirring at room temperature, and reflux for 4. 5 h. Pour into 10% hydrochloric acid (500 ml) at 0°C, separate the organic layer, extract the aqueous layer with benzene (200 ml×2), combine the organic layers, wash with saturated sodium bicarbonate solution and water until neutral, and use Dry over hydrated magnesium sulfate and distill under reduced pressure. Collect the 130~134°C/4.27 kPa fraction to obtain 1 (43.2 g, 99.1%), mp 55.5~56°C (document yield 76%, mp 55. 5~57. 3℃). The purity is greater than 99% (online determination of color and quality).
Method 2: Add benzoic acid (BA), cyclohexylcarboxylic acid (CCA) and catalyst to the autoclave, and introduce nitrogen to exhaust the air in the autoclave. Close the ventilation valve, turn on the stirrer, and start heating. When it reaches the specified temperature, maintain this temperature and continue the reaction. After the reaction is completed, take out the autoclave furnace from the furnace jacket and put it into cold water to cool to room temperature. Open the autoclave, take out the materials, add acetone, and oscillate in a sonic oscillator 10 minutes
Fully dissolve the organic phase, filter the composition through a Buchner funnel, and calculate the yield. Filter with shaking for 3 times, use liquid chromatography to analyze the composition of the filtrate, and calculate the yield. The relatively pure cyclohexyl phenyl ketone can be obtained by distilling the filtrate under reduced pressure. In the reaction where manganese salt or oxide is the catalyst, benzoic acid, cyclohexylcarboxylic acid and the catalyst generate carboxylic acid manganese salt C6H5COO -Mn -OOCC6H11, and the product cyclohexyl phenyl ketone is mainly generated by high-temperature pyrolysis of carboxylic acid manganese salt. The reaction is a free radical process, and the mechanism is as follows:

Main ReferencesMaterial
[1] Synthesis of cyclohexyl phenyl ketone
[2] Research on one-step synthesis of cyclohexyl phenyl ketone
[3] CN201810727567.7 Preparation process of photoinitiator 184
[4] CN201610800768.6S-α-cyclohexylbenzylamine
[5] One-step synthesis of cyclohexyl phenyl ketone

 微信扫一扫打赏
微信扫一扫打赏

