Background and Overview
3-Bromodibenzo[B,D]furan is a chemical intermediate.
Preparation[1]
Synthesis of 3-nitrodibenzofuran:
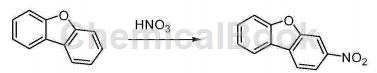
Add 150ml trifluoroacetic acid to the reaction bottle, then add dibenzofuran (14.2g, 82mmol), stir vigorously at room temperature to dissolve it, cool it in ice-water bath, and add 70% HNO3 (9.1g, 101mmol) to 50ml Trifluoroacetic acid, slowly added to the reaction bottle, stirred for 40 minutes, then poured the reaction solution into 300ml ice water and stirred for 30 minutes, filtered, to obtain an off-white solid, washed with 2M sodium hydroxide solution and water, respectively, to obtain the solid Then recrystallize with ethanol to obtain 14.7g of light yellow solid.
Synthesis of 3-aminodibenzofuran:
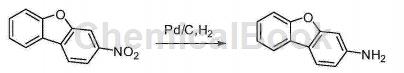
3-Nitrodibenzofuran (12.6g, 60mmol) was dissolved in 800ml ethyl acetate, purged with nitrogen for 5 minutes, 1000mg Pd/C was added, hydrogenation started, pressurized hydrogen 60psi, when 60psi The reaction is complete when the pressure can be maintained stably for 15 minutes and no longer decreases. The reaction solution is filtered with a sand core funnel, and the filtrate is spin-dried to obtain a white solid (10.9g, 58mmol).
Synthesis of 3-bromodibenzofuran:

Sodium nitrite (4.4g, 65mmol) was dissolved in 40ml of concentrated sulfuric acid at ℃, and 3-aminodibenzofuran (10.6g, 58mmol) was dissolved in a small amount of glacial acetic acid and slowly dripped into the reaction solution, keeping The temperature is lower than 5°C. After the dropwise addition, keep it at 0°C and continue stirring for 2 hours. Add 200ml of ether to the reaction solution and stir. Diazonium salt will precipitate. Filter to obtain a brown solid; add CuBr (12.5g) to another reaction bottle. , 87mmol), 300ml 48% HBr, finally add the obtained brown diazonium salt, heat to 66°C for 2 hours, cool to room temperature, filter, wash the filter cake with water twice, and use petroleum ether: dichloromethane to obtain the solid =10:1 eluent was passed through the column to obtain 9.6 g of solid.
Apply [1]
CN201510906440.8 reported that 3-bromodibenzo[B,D]furan can be used to synthesize a phosphorescent iridium complex 2 containing a 3-phenylpyridazine structure. This iridium complex uses metallic iridium as the core and as the phosphorescent doping material of the light-emitting layer. The OLED device can achieve high brightness, high efficiency, and low voltage effects. The reaction is divided into three steps as follows:
Synthesis of 2-(3-dibenzofuran)pinacolborane:
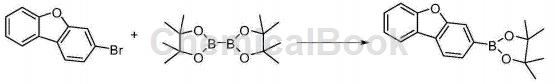
3-Bromodibenzofuran (4.8g, 19.4mmol), bispinazol diborane (6.5g, 25.5mmol), KOAc (7.7g, 78mmol), and 100ml dioxane were added to the reaction bottle. , purge nitrogen for 10 minutes, add Pd(dppf)2Cl2 (160mg, 0.02mmol), purify nitrogen for another 10 minutes, heat to 80°C overnight for about 20 hours, naturally cool to room temperature, filter, wash the filtrate with water, and extract with ethyl acetate , the organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was spun dry to obtain a brown solid, which was passed through a column to obtain 5.1 g of solid.
Synthesis of 2-(3-dibenzofuran)pyridine:
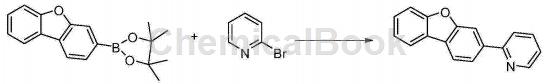
Dibenzofuran pinacol borate (11.8g, 40mmol), 2-bromopyridine (5.8ml, 60mmol), sodium carbonate (10.2g, 96mmol), tetrakis(triphenylphosphine)palladium (0.5 g, 0.4 mmol), 50 ml each of toluene, ethanol, and water were added to the reaction bottle in sequence, and the reaction was refluxed under nitrogen protection for 10 hours. Cool to room temperature, separate the liquids, extract the aqueous layer with EA, combine the organic layers, and use saturated saline and saturated saline respectively. Wash with water, dry the organic layer over magnesium sulfate, filter, spin the filtrate to dryness, and pass through a silica gel column. The eluent ratio is petroleum ether: ethyl acetate = 20:1 to obtain 8.7g of product, with HPLC purity of 99.0%.
Synthesis of compound 2:
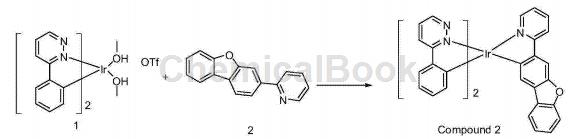
Put raw material 1 (2.9g, 4.0mmol) and raw material 2 (4.1g, 16.2mmol) into the reaction bottle, add 70ml ethanol, and reflux for 24 hours under nitrogen protection. A yellow solid precipitates during the reaction. After the reaction is completed Filter, and the obtained bright yellow solid passes through the column. The eluent ratio is petroleum ether: dichloromethane = 1:1, and 1.1 g of solid is obtained, which is the compound 2, with a purity of 99.2%.
Main reference materials
[1] CN201510906440.8 A phosphorescent iridium complex containing 3-phenylpyridazine structure and its application

 微信扫一扫打赏
微信扫一扫打赏

