Background and overview[1][2]
2,3-Dichloroaniline is an important organic raw material intermediate. 2,3-Dichloroaniline is widely used in fine chemical industries such as pesticides, medicines, dyes and photosensitive materials. Regarding the synthesis line of 2,3-dichloroaniline, it mainly uses 2,3-dichloronitrobenzene as the raw material. Some studies have mentioned that tungsten carbide is used as the catalyst and hydrogenation reduction is obtained. The main problem is that the hydrogenation process affects The equipment material requirements are high and there are certain safety hazards; secondly, the preparation and purification route of 2,3-dichloronitrobenzene is relatively complicated, resulting in high raw material costs.
Structure[1]
2,3-Dichloroaniline is an important organic raw material intermediate, such as the photodegradable impurity of aripiprazole in the synthesis of aripiprazole according to the present invention. Aripiprazole, chemical name is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2( 1H)-Quinolinone, developed by Otsuka Pharmaceutical Co., Ltd. of Japan and launched in the United States in November 2002, is a third-generation atypical antipsychotic drug. Aripiprazole is the first dopamine system stabilizer and has significant effects on both positive and negative symptoms of schizophrenia. . Aripiprazole is used to treat schizophrenia and can significantly improve the symptoms of schizophrenia without some of the common side effects of other antipsychotics, such as weight gain and involuntary muscle movements. Aripiprazole photodegradation impurities refer to impurities produced by degradation under light conditions. The synthesis method is: using 2,3-dichloroaniline as raw material, reacting with compound V to generate compound II, and compound II reacting with compound III to obtain The target product compound I. A in the compound V is Cl, Br, COOH, CHO or COCl group; A’ is NH2 or NO2 group; n=1 or 2. The preparation method for photodegrading impurities of aripiprazole has a reasonable synthesis route, readily available raw materials, simple and easy operation, and the obtained target product has high yield and high purity.
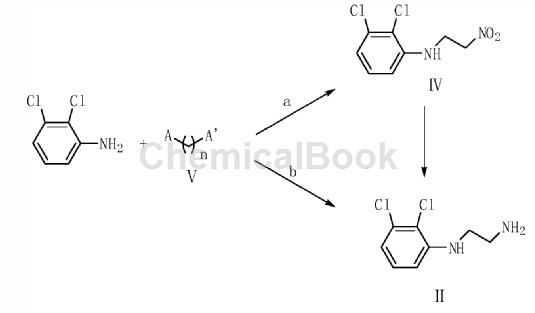
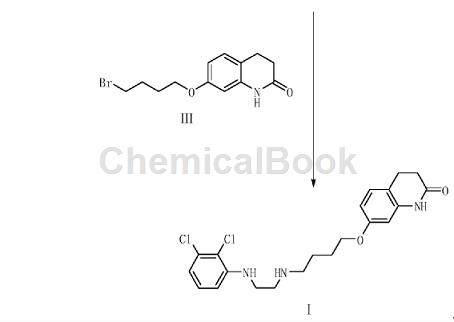
Preparation [2]
A preparation method of 2,3-dichloroaniline/2,6-dichloroaniline. The process route of this method uses 1,2,3-trichlorobenzene, a by-product in the production process of dichlorobenzene, as raw material. Ammonolysis yields 2,3-dichloroaniline/2,6-dichloroaniline. During the process, a catalytic amount of catalyst and cocatalyst is added, while the addition of triethylamine is avoided. The process is simple, low cost, and has less “three wastes” and other advantages. The process of preparing 2,3-dichloroaniline/2,6-dichloroaniline through aminolysis of 1,2,3-trichlorophenyl is a nucleophilic substitution reaction, since there is no strong electron-withdrawing group on the benzene ring of trichlorophenyl ( Such as nitro, sulfo or cyano), resulting in -Cl not being active enough. In addition, the ammonolysis process is a two-phase reaction of oil and water. Catalysts and cocatalysts need to be added to carry out catalytic ammonolysis under certain temperature and pressure conditions in an autoclave. The technical solution is: put ammonia water and 1,2,3-trichlorobenzene into the reactor in a certain proportion, control the appropriate reaction temperature and pressure, carry out the ammonolysis reaction under the action of the catalyst and cocatalyst, and cool down and release the pressure after the reaction is completed. The obtained reactants are then analyzed and separated to obtain 2,3-dichloroaniline and 2,6-dichloroaniline finished products. The concentration of ammonia water is 10%-60%, preferably 30%-50%. The molar ratio of 1,2,3-trichlorobenzene to ammonia is 1:6.0-30.0, preferably 1:8.0-16.0. The catalyst for ammonolysis is a metal ion capable of complexing with ammonia, such as copper ions, manganese ions, cobalt ions, etc., preferably metal copper ions. The catalyst can be added alone or pre-dissolved in ammonia water. The usage amount of the catalyst is 0.1%-10% of the molar amount of 1,2,3-trichlorobenzene, preferably 1.0%-5.0%. The cocatalyst is a phase transfer catalyst, used to improve the mass transfer between the organic phase and ammonia water, and increase the ammonolysis yield. Phase transfer catalysts are quaternary ammonium salts and polyethers, and the weight ratio of dosage to catalyst is 1:1. The reaction temperature is 100°C-250°C, the pressure range is 2.0-10.0MPa, preferably 150°C-210°C, the pressure range is 3.0-7.0MPa. The aminolysis reaction time is 5-60h, preferably 10-50h. The main reaction equation is as follows (taking cuprous ions as an example):
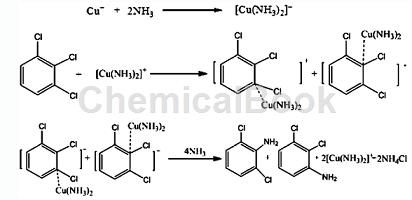
Using 1,2,3-trichlorobenzene and ammonia as raw materials, metal copper ions as catalysts, quaternary ammonium salts and polyethers as phase transfer catalysts, 2,3-trichlorobenzene is prepared by ammonolysis under certain temperature and pressure conditions. Chloroaniline/2,6-dichloroaniline. Compared with the traditional 2,3-dichloroaniline/2,6-dichloroaniline process, the advantage of this invention is that it uses 1,2,3-trichlorobenzene, a by-product in the production process of dichlorobenzene, as raw material and performs one-step ammonialysis 2,3-dichloroaniline/2,6-dichloroaniline is obtained, which has the advantages of simple process flow, low equipment investment, high atom utilization rate, and less “three wastes”.
Main reference materials
[1] CN201611077656.9 Preparation method of aripiprazole photodegradable impurities
[2] CN201410220617.4 A method for preparing 2,3-/2,6-dichloroaniline

 微信扫一扫打赏
微信扫一扫打赏

