Background and overview[1][2]
Pyrazolin-5-one is an important class of heterocyclic compounds with physiological effects. It can be used as an antipyretic, analgesic, antibacterial agent, etc. It can also be used as an analytical reagent, an antioxidant for rubber, linseed, and textiles. Dyes, etc. 4-aryl methylene-3-methyl-1-phenyl-5-pyrazolone and 4,4′-aryl methylene-bis(1-phenyl-3-methyl-5-pyrazoles) Linones) are important intermediates in organic synthesis. The C-4 methylene substitution of 3-methyl-1-phenyl-5-pyrazolone is widely used in photography and can be used as photosensitizer, supersensitizer, developer, etc. 4,4′-aryl methylene-bis(1-phenyl-3-methyl-5-pyrazolone) can also be used as an extraction agent for radioactive elements such as uranium, and can also be used as a ligand. 1-phenyl-3-methyl-5-pyrazolone can be used as a raw material for preparing 4,4′-arylene-bis(1-phenyl-3-methyl-5-pyrazolone).
1-Phenyl-3-methyl-5-pyrazolone is an important intermediate, light yellow crystalline powder. At present, most industries use the acetoacetamide aniline method. Since the production process requires a large amount of sulfuric acid and ammonia gas, the process route is long, and a large amount of wastewater and waste gas containing acid and amines are produced, which causes great environmental pollution and is technically backward. Some studies have synthesized l-phenyl-3-methyl-5-pyrazolinone by adding phenylphosphorus solution dropwise in ethanol solvent of ethyl acetoacetate. This method has a short production process and high product yield. High, high purity.
Apply[1-6]]
Pyrazolin-5-one is an important class of heterocyclic compounds with physiological effects. It can be used as an antipyretic, analgesic, antibacterial agent, etc. It can also be used as an analytical reagent, an antioxidant for rubber, linseed, and textiles. Dyes, etc. Examples of its application are as follows:
1. Preparation of pyrazolin-5-one compounds.
Under solvent-free and catalyst-free conditions, microwave-promoted condensation reaction of 1-phenyl-3-methyl-5-pyrazolone and aromatic aldehyde will yield 4-aryl methylene-3- Methyl-1-phenyl-5-pyrazolone and 4,4′-aryl methylene-bis(1-phenyl-3-methyl-5-pyrazolone) in good yields.
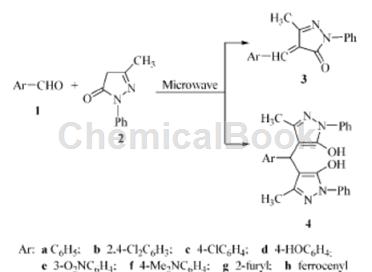
1) Synthesis of 4-arylmethylene-3-methyl-1-phenyl-5-pyrazolinone
Place 0.5 g (2.9 mmol) of 1-phenyl-3-methyl-5-pyrazolinone and 5.8 mmol of aromatic aldehyde in a small beaker, mix evenly, cover with a watch glass, and place in a microwave oven . Select appropriate power and time for irradiation, cool, and recrystallize with CHCl3-CH3OH to obtain 3a ~ 3h.
2) Synthesis of 4,4′-aryl methylene-bis(1-phenyl-3-methyl-5-pyrazolinone)
Place 0.5 g (2.9 mmol) of 1-phenyl-3-methyl-5-pyrazolinone and 3 mmol of aromatic aldehyde in a small beaker, mix evenly, cover with a watch glass, and place in a microwave oven .Choose appropriate power and time to irradiate, cool, and recrystallize with CHCl3-CH3OH to obtain 4a ~ 4e.
2. Clean production process for preparing a solvent yellow BL,
After anthranilic acid is dissolved in hydrochloric acid, sodium nitrite is added to carry out diazotization reaction, and the obtained diazo liquid is added to the coupling liquid of 1-phenyl-3-methyl-5-pyrazolinone. The coupling reaction lasted for 2 hours, and the obtained azo compound solution was directly subjected to the complexation reaction without filtering, washing, and drying. Add acid-binding agent and complexing agent CRF to the obtained azo compound solution and stir evenly. Add a pressure kettle to heat the reaction. After cooling to normal temperature, filter and wash with water to obtain complex B. Through this production process, the cost of raw materials and the cost of treating three wastes can be reduced, making the solvent yellow BL a green and environmentally friendly product.
3. Prepare Solvent Orange 2A.
Solvent Orange 2A is mainly used for wood dyeing, coloring of various natural and synthetic leathers, and coloring of various metal transparent coatings, solvent-based inks, aluminum foil, vacuum electroplating films and hot stamping materials.
The production of Solvent Orange 2A uses 4-nitro-2-aminophenol and 1-phenyl-3-methyl-5-pyrazolinone as the basic raw materials. The process steps include coupling reaction, complexation reaction and substitution. Reaction steps, the complexation reaction steps are as follows: add amide mixed solvent to alcohol ether solvent and complexing agent CRF, heat to 100°C, add azo compound A, directly heat to 138-150°C, reaction 2.5- 3.5 hours, cool down and precipitate into water, filter and wash with water to obtain complex B. The dosage of the mixed solvent of the present invention is more than half less than that of the alcohol ether solvent in the original process, and the unit price is low and extremely efficient.��Reduced production costs.
4. As a pickling corrosion inhibitor.
Corrosion inhibitors have stood out in the field of pickling and anti-corrosion and become a focus of academic frontiers. Some antibacterial alkaloids, fungicides, vitamins, amino acids and plant-extracted organic acids have been studied and proven to be able to produce competitive adsorption reactions on metal surfaces and corrosive media such as acidic or neutral solutions, and work has been done to gradually screen them out, such as: Compounds such as purines, amino acids, ciprofloxacin and levofloxacin all have the characteristics of maintaining high corrosion inhibition efficiency in acidic environments such as sulfuric acid and hydrochloric acid.
The active ingredient of the corrosion inhibitor is 1-phenyl-3-methyl-5-pyrazolone. The corrosion inhibitor of the present invention can prevent the corrosion electrochemical reaction (local corrosion and general corrosion) of carbon steel materials and their products when they come into contact with sulfuric acid or hydrochloric acid solutions. It has a small dosage, a wide range of sources, is non-toxic and has high efficiency. , has strong continuous action ability, can effectively inhibit the corrosion damage of carbon steel, and has significant application value and broad market prospects.
5. Prepare solvent yellow BL.
The production of solvent yellow BL uses anthranilic acid and 1-phenyl-3-methyl-5-pyrazolinone as basic raw materials, and the process steps include coupling reaction, complexation reaction and substitution reaction steps. The complexation reaction steps are as follows: heat the amide mixed solvent and complexing agent CRF to 100°C, add azo compound A, directly raise the temperature to 138-150°C, react for 2.5-3.5 hours, cool down and precipitate into water, filter and wash with water Complex B is obtained; the product quality can be improved through this production process, and the impurity content of solvent yellow BL is reduced by 50% compared with the original. The amount of amide solvent is more than half less than that of alcohol ether solvent in the original process, and the unit price Low, greatly reducing production costs.
Preparation [1]
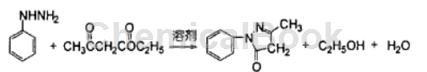
In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser and a thermometer, add aniline 16.2 9 (0.15 mol) and 50 ml of absolute ethanol. When stirring and heating to 60°C, start adding acetoacetic acid dropwise. 20.5g (0.1575 mol) of ethyl ester was added dropwise within 1 to 5 hours. At this time, the reaction temperature was controlled between 60 ℃ and 75 ℃. After the dripping was completed, the reaction was maintained and stirred under reflux for 7 hours. The reaction was stopped and the solvent was distilled under reduced pressure. 25 ml of ethanol, cooled, crystallized, and a light yellow solid precipitated. Recrystallize the crude product twice with absolute ethanol and dry it to obtain a 1-phenyl-3-methyl-5-pyrazolone product with a purity of >99% and a melting point of 127 ℃ -128 ℃.
Main reference materials
[1] Research on the synthesis process of 1-phenyl-3-methyl-5-pyrazolinone
[2] Study on the reaction of 1-phenyl-3-methyl-5-pyrazolinone and aromatic aldehydes under the promotion of microwave without solvent and catalyst
[3] CN201710724203.9 A clean production process of solvent yellow BL
[4] Production process of CN200610096541.4 Solvent Orange 2A
[5] CN201610867556.X Application of a pyrazolone compound as a pickling corrosion inhibitor
[6] Production technology of CN200610096542.9 solvent yellow BL

 微信扫一扫打赏
微信扫一扫打赏

