Background and overview[1]
2,4-Difluorobiphenyl is not only an important intermediate and the key to the synthesis of fluorobenzene salicylic acid, it can also be used as an anti-overcharge additive for battery electrolytes, and its purity is the key to the synthesis of fluorobenzene salicylic acid. Key to preventing overcharging of batteries and their performance. Lithium batteries include positive electrodes, negative electrodes, electrolytes and separators. However, during the charging and discharging process, the battery will release heat, resulting in reduced battery performance. At the same time, there is also the phenomenon of overcharging of the battery, which has a negative impact on the development of lithium-ion batteries and the electronic information industry. create constraints. The existing method is to add battery additives to prevent the harm and disadvantages caused by overcharging of batteries. 2,4-Difluorobiphenyl is such an overcharge prevention additive.
Preparation[1]
There are currently two main methods used in the preparation of 2,4-difluorobiphenyl. Method 1 uses Gomberg-Bachmann coupling reaction. This method has many advantages, but low yield is the biggest problem encountered in industrial production. Method 2 uses 2, 4-dinitroaniline as raw material and undergoes diazotization, coupling, reduction, diazotization and pyrolysis to prepare 2,4-difluorobiphenyl. This method has lengthy steps and poor overall yield. Low. Therefore, how to improve the yield of 2,4-difluorobiphenyl is an urgent technical problem that needs to be solved.
CN201710202399.5 provides a synthesis method of 2,4-difluorobiphenyl to solve the problems of low yield, complex operation and lengthy reaction steps of 2,4-difluorobiphenyl. The technical solution adopted by this invention to achieve its purpose is:
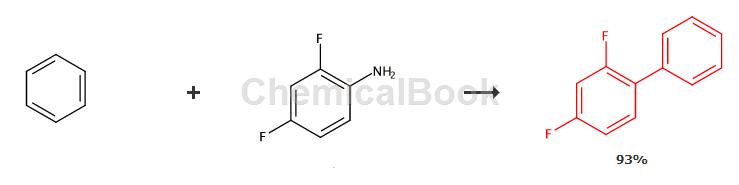
The synthesis method of 2,4-difluorobiphenyl uses 2,4-difluoroaniline and benzene as raw materials, diazotizes 2,4-difluoroaniline and performs a coupling reaction with benzene to obtain 2, 4-Difluorobiphenyl, add trifluoroacetic acid, anhydrous magnesium sulfate, catalyst and benzene to the reaction kettle, stir and cool to 2-6°C, add isoamyl nitrite, control the temperature to 6-10°C, and add Add 2,4-difluoroaniline dropwise, and control the reaction temperature to be 10-25°C during the dropwise addition. After the addition is completed, stir and react at 5-12°C for 1-3 hours. After the reaction is completed, the reaction mixture is filtered, washed, Dry and distill under reduced pressure to obtain 2,4-difluorobiphenyl.
The effective effects of the present invention are:
The synthesis method of the present invention is simple, easy to operate, the reaction process is simple, and the procedures are short. The purity of the synthesized 2,4-difluorobiphenyl reaches more than 99.3%, and the yield reaches more than 88%.
The present invention uses isoamyl nitrite as the diazotizing agent, and adds trifluoroacetic acid to the system, so that the diazonium salt generated during the reaction can be well dissolved in the organic solvent, so that the reaction can be carried out in a homogeneous medium in progress. By controlling the amount of raw materials used and the order in which each raw material is added, the entire reaction is made mild, high-temperature reactions are avoided, and by-products are reduced.
The composite catalyst of the present invention can improve the purity and yield of 2,4-difluorobiphenyl, and the catalyst is easy to obtain, which provides a basic guarantee for the preparation of 2,4-difluorobiphenyl with high purity and high yield. The control of the proportion of the composite catalyst is the guarantee for the stable progress of the reaction. After long-term creative research, the catalyst with this proportion has good catalytic effect, can reduce the progress of side reactions, and promote the rapid and stable decomposition of diazonium salts necessary for coupling. of aromatic hydrocarbon radicals.
The function of anhydrous magnesium sulfate is to remove the water produced during the reaction, making the reaction system homogeneous, and at the same time pushing the reaction toward the production of the target product.
Main reference materials
[1] CN201710202399.5 Synthesis method of 2,4-difluorobiphenyl

 微信扫一扫打赏
微信扫一扫打赏

