Background and overview[1][2]
(2-Chloro-5-iodophenyl)(4-fluorophenyl)methanone is an intermediate in the synthesis of empagliflozin. Empagliflozin is a type 2 sodium-glucose cotransporter inhibitor jointly developed by Boehringer Ingelheim and Eli Lilly and Company. SGLT-2 inhibitor is a new type of hypoglycemic drug. It mainly inhibits SGLT-2 expressed in the kidney, reduces renal glucose reabsorption, increases the excretion of glucose in urine, thereby reducing plasma glucose levels. Its hypoglycemic effect is not good. Dependent on beta cell function and insulin resistance. This product was first approved for marketing by the European Medicines Agency (EMA) in May 2014 and approved by the US FDA in August 2014 for the treatment of type 2 diabetes. Its empagliflozin/linagliptin combination tablet is the first combination antidiabetic drug containing an SGLT-2 inhibitor and a dipeptidyl peptidase-4 inhibitor.
Preparation[1]
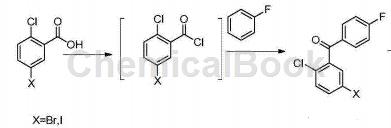
Add (282g, 1mol) 5-iodo-2-chlorobenzoic acid, 1.0L dichloromethane, (142.8g, 1.2mol) thionyl chloride and catalytic amount of DMF into the reaction bottle, and heat it to 39℃-42 ℃ reaction 3hrs-5hrs. Cool the temperature to 5-10°C, add (160g, 1.2mol) aluminum trichloride and (125g, 1.3mol) fluorobenzene, and heat to reflux overnight. After the reaction is completed, cool to 0°C, add 100ml 6N hydrochloric acid dropwise to quench the reaction, add 400ml water to separate the organic phase, wash the organic phase with 400ml saturated sodium bicarbonate solution, 400ml saturated sodium chloride solution, dry over anhydrous sodium sulfate, and concentrate to Dry, add 100 ml of absolute ethanol, heat to dissolve, cool to 5-10°C, incubate for 2 hours to crystallize, filter with suction, and dry to obtain a white solid (5-iodo-2-chlorophenyl) (4-fluorophenyl) methanone 310g.
The beneficial effects of the present invention are reflected in:
(1) The present invention replaces the ethoxy metal reagent with cheap inorganic bases (KOH, NaOH, LiOH) and uses the reactant ethanol itself as the solvent, thus avoiding the use of high boiling point solvents (DMSO and DMF). On the one hand, the use of inorganic bases (KOH, NaOH, LiOH) and ethanol effectively reduces the cost of the reaction; on the other hand, the reaction solvent is easier to recycle and reuse, effectively reducing the COD in the wastewater, making it more green and environmentally friendly;
(2) The preparation method of the present invention utilizes Empagliflozin’s (5-halo-2-chlorophenyl)(4-fluorophenyl)methanone, which can integrate production resources well and can further reduce the cost of production cost purposes.
Apply[2]
Intermediate II is an important intermediate in the synthesis of SGLT2 inhibitors. (2-Chloro-5-iodophenyl)(4-fluorophenyl)methanone can be used to synthesize Intermediate II.
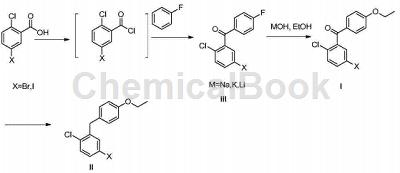
Synthesis of intermediate I-(5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanone:
Add (18g, 50mmol) (5-iodo-2-chlorophenyl) (4-fluorophenyl) methanone, 100ml absolute ethanol and (6.8g, 100mmol) sodium ethoxide into the reaction bottle, and heat to 50 React at ℃-65℃ for 1 hour. TLC monitors the reaction progress. There are obvious impurities generated; continue the reaction for 3 hours until the raw materials basically disappear. Pour the reaction solution concentrated to 20-30ml under reduced pressure into ice water, precipitate the solid, filter, filter The cake was washed with water, dried, and passed through a column to obtain 8.1 g of product compound I (5-iodo-2-chlorophenyl) (4-ethoxyphenyl) methanone, with a yield of 42%.
Intermediate II-5-iodo-2-chloro-4′-ethoxydiphenylmethane:
Add (386.6g, 1mol) (5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanone and 1.5L THF obtained according to the method of Example 3 into the reaction bottle, and stir the solid Completely dissolve, lower the temperature to 0°C under nitrogen protection, add (41.6g, 1.1mmol) sodium borohydride, stir for 30 minutes, then control the temperature to below 10°C and add aluminum trichloride in batches, stir for about 30 minutes after addition, and raise the temperature to reflux reaction ; After the reaction is completed, lower the temperature and drop hydrochloric acid solution into the reaction bottle to quench the reaction, add EA for extraction, wash the organic phase with saturated brine, dry with anhydrous sodium sulfate, and spin dry the solvent under reduced pressure; add 120 ml of ethanol, cool to about 0°C to precipitate a solid , filter, wash the filter cake with a small amount of ice ethanol, and dry to obtain 339.8g of compound II 5-iodo-2-chloro-4′-ethoxydiphenylmethane, with a yield of 91.2% and an HPLC purity of 99.7%.
Main reference materials
[1] CN201810396954.7 Preparation method of SGLT2 inhibitor intermediate

 微信扫一扫打赏
微信扫一扫打赏

