Background and overview[1]
Meta-aminobenzene sulfonamide can be used as an intermediate for pharmaceutical and chemical synthesis. If meta-aminobenzene sulfonamide is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if contact with eyes, seek medical attention. Separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
Apply[1]
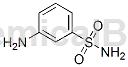
Meta-aminobenzene sulfonamide can be used as an intermediate for pharmaceutical and chemical synthesis. If the following reaction occurs:
The specific steps are: add m-aminobenzenesulfonamide (4.98g, 24mmol) and 4-chloro-6-(2-benzyloxyphenyl)-pyrimidine (6.88g, 23.2mmol) in DMFA (50mL) The mixture was stirred at 80 °C. until the reaction is complete (TLC control). The reaction mixture was diluted with water (200 mL) and treated with NaHCO3 (4.2 g, 50 mmol). The resulting precipitate was filtered off, washed with water (2 × 200 mL), dried and purified by silica gel column chromatography (eluent chloroform-ethanol 20:1) to give [6-(2-benzyloxy-phenyl)-pyrimidine -4] -(3-Methanesulfonyl-phenyl)-amine, colorless solid. Yield: 8.10g (81%).
Preparation[1]
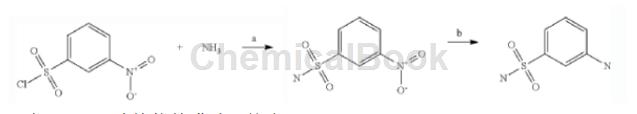
Step a: Synthesis of 3-nitrophenylsulfonamide
Dissolve 3-nitrophenylsulfonyl chloride (4.71 g, 20 mmol) in acetonitrile (20 mL), then add concentrated aqueous ammonia saturated with ammonium carbonate (20 mL) to this solution and mix the reaction mixture Stir vigorously for 1 hour. in room temperature. The acetonitrile was then evaporated and the residue was diluted with cold water (20 mL), which resulted in the formation of a precipitate. The precipitate was filtered off and washed with water (2×5 mL) and diethyl ether and dried under reduced pressure. Yield of 3-nitrophenylsulfonamide 3.5 g (80%)
���Step b: Synthesis of m-aminobenzenesulfonamide
Hydrogenate 3-nitrobenzenemethanesulfonamide (3.5G, 16 mmol) in a solution of Raney nickel (0.5 g) in methanol at 50°C and 70 PSI for 4 hours, then filter off the catalyst and rinse with warm methanol Wash and evaporate the combined filtrates to give 2.83 g (95%) of m-aminobenzenesulfonamideamine.
Main reference materials
[1] US20110306602. 4, 6-disubstituted aminopyrimidine derivatives as inhibitors of protein kinases

 微信扫一扫打赏
微信扫一扫打赏

