Background and overview[1][2]
4,6-Diaminoresorcin dihydrochloride is an important chemical intermediate and a monomer for the synthesis of polymer material polybenzobisoxazole (PBO). PBO is a super-performance fiber in the 21st century. It has very excellent physical, mechanical and chemical properties. Its strength and modulus are twice that of Kevlar fiber. It also has the heat-resistant and flame-retardant properties of meta-aramid fiber, and its physical and chemical properties completely exceed Kevlar fiber is by far the leader in the field of high-performance fibers. A PBO filament with a diameter of 1 mm can lift a weight of 450 kilograms, and its strength is more than 10 times that of steel wire fibers.
Preparation[1]
U.S. Patent No. 412246 discloses a method to prepare 4,6-diaminoresorcin from resorcin through chloroformate alkylation, mixed acid nitration, catalytic hydrogenation and other processes. The disadvantage of this method is that the reaction The product concentration is not high and requires impurity separation and multiple purifications, which is detrimental to reducing product costs. US patent US4745232 reports that 4,6-dinitroresorcin is prepared through direct nitration of resorcin, and 4,6-diaminoresorcin is prepared through catalytic hydrogenation. However, the product purity of this method is low and the yield is low. Not high and difficult to produce industrially.
The above-mentioned processes using resorcinol as raw materials all adopt sulfonation and nitration processes, and all use fuming sulfuric acid, which places high requirements on equipment and operators. Moreover, catalytic hydrogenation reduction is used in the nitro reduction reaction. In this process, precious metal catalysts such as palladium are used, and high-pressure hydrogenation equipment is used, which is costly.
CN201110257600.2 provides a preparation method of 4,6-diaminoresorcinol and its hydrochloride. The specific steps are: 1) In the nitrification reactor, add 98% concentrated sulfuric acid as the base material, then add resorcinol and stir to dissolve it, then add dropwise mixed acid containing sulfuric acid and nitric acid to treat resorcinol at 35-75°C. The diphenol is nitrated, react for 2 to 5 hours to obtain a nitrated mixture, and then heat and dissolve the nitrated mixture in a solvent, cool, and then recrystallize to obtain 4,6-dinitroresorcinol; 2) Combine the nitrated mixture obtained in step 1) Add 4,6-dinitroresorcinol into the reactor, then add an organic solvent to dissolve it, then add a reducing agent to react with 4,6-dinitroresorcinol at 45~95°C for 2~ 6h; cool down and let stand, filter to obtain the crude product, then dissolve it in deoxygenated water, then add activated carbon to the deoxygenated water to decolorize the crude product at 50-100°C, filter, and then add concentrated hydrochloric acid dropwise to the filtrate to obtain white color The crude product of 4,6-diaminoresorcin dihydrochloride is crystallized, and then recrystallized with hydrochloric acid to obtain pure 4,6-diaminoresorcin dihydrochloride.
The reaction conditions of the present invention are mild, and the intermediate 4,6-dinitroresorcinol is easily soluble in alcohol solvents such as methanol and ethanol, making nitro reduction easier; the requirements for equipment and personnel are low, and the cost is Low.
Apply [2]
For the preparation of polybenzoxazole:
PBO fiber – the abbreviation of Cis-Polyparaphenylene benzobi-soxazole (abbreviated as PBO) fiber. The fiber has a tensile strength of 575kg/cm2, an initial modulus of 28000kg/cm2, a limiting oxygen index LOI value of 68, and a decomposition temperature of 650°C. The performance indicators are among the best among organic and inorganic fibers at present. Its specific strength is about 12-14 times that of steel, its specific modulus is 1.4 times that of steel, and its specific gravity is 1.52-1.55g/cm2Only 1/5 of steel. Due to its superior performance, it has been regarded as a new generation of super fiber for advanced structural composite materials such as aerospace and military since its advent. PBO uses 4,6-aminoresorcin hydrochloride and terephthalic acid (TA), polyphosphoric acid (PPA) and phosphorus pentoxide (P2O 5) is prepared by solution condensation polymerization using a solvent. PPA is both a solvent and a polycondensation catalyst. Synthetic route:
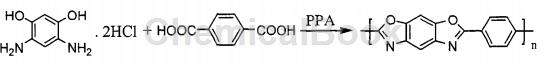
The entire polymerization process is divided into two stages. First, 4,6-diaminoresorcin dihydrochloride monomer removes HCl, and then reacts with terephthalic acid to prepare PBO with a certain viscosity through polycondensation reaction. slurry, and then through dry-jet wet spinning to prepare PBO fibers. This method has the following deficiencies: First: during the removal of hydrochloric acid by 4,6-diaminoresorcin hydrochloride, a large amount of hydrogen chloride gas is released, and these gases will��A large amount of foam is generated in the viscous reaction liquid, thereby reducing the stirring efficiency of the reaction system. Second: Hydrogen chloride is a highly corrosive gas, which makes the reaction very demanding on equipment and materials, and the degassing process takes up a lot of time. Third: During the process of dehydrochlorination gas, a large amount of foam is produced, causing 4,6-diaminoresorcin hydrochloride to adhere to the reactor wall, destroying the equimolar ratio of the two monomers, which is serious. affects the final molecular weight of the polymer. Fourth, it is difficult to completely remove hydrochloric acid in the prior art. Therefore, phosphorus pentoxide is usually added in a distributed manner. In order to facilitate degassing, the temperature of the reaction system must be increased. However, under high temperature conditions, It will lead to an increase in side reactions and is not conducive to an increase in molecular weight.
US patent US5276128 reports a method to overcome the above problems, that is, terephthalic acid is formulated into an aqueous solution of terephthalic acid sodium salt, and 4,6-diaminoresorcin dihydrochloride is formulated into an aqueous solution. Acid-base neutralization reaction, filtration, washing and drying are carried out to prepare 4,6-diaminoresorcin/terephthalate, and then polymerization reaction is carried out. The disadvantages of this method are: first, 4,6-diaminoresorcin hydrochloride is relatively easy to oxidize, so when preparing the aqueous solution, the water needs to be oxygen-free and protected with nitrogen; second, through acid-base neutralization Afterwards, it needs to be filtered and washed, so that the prepared 4,6-diaminoresorcin/terephthalate is easily exposed to the air and oxidized, and it needs to be dried under vacuum conditions, which is time-consuming and labor-intensive.
CN200510026800.1 provides a preparation method for polybenzoxazole, which improves the salt-forming process and directly adds solid 4,6-diaminoresorcin hydrochloride into the reaction system to perform the salt-forming reaction. , avoiding the need to use anaerobic water and nitrogen protection when preparing aqueous solutions; there is no need to wash and dry the composite salt obtained through acid-base neutralization, saving time and eliminating the need to remove hydrogen chloride, thereby overcoming the background problem Problems in technology. Achieved through the following technical solution, terephthalic acid and 4,6-diaminoresorcin dihydrochloride are directly neutralized by acid and alkali to form 4,6 diaminoresorcin/terephthalic acid composite salt , directly add phosphorus pentoxide into this system to polymerize 4,6 diaminoresorcin/terephthalic acid composite salt to prepare polybenzoxazole, realizing the integration of salt formation and polymerization, simplifying the reaction process, eliminating the cumbersome post-processing process of compound salt and improving efficiency.
Main reference materials
[1] CN201110257600.2 Preparation method of 4,6-diaminoresorcinol and its hydrochloride
[2] Preparation method of CN200510026800.1 polybenzoxazole

 微信扫一扫打赏
微信扫一扫打赏

