Background and overview[1]
3-Bromo-5-methoxybenzaldehyde is an aldehyde compound that can be used as an intermediate in organic synthesis.
Structure
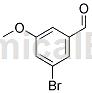
Apply [1]
3-Bromo-5-methoxybenzaldehyde can be used as an intermediate in organic synthesis. For example, prepare the following compounds:
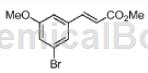
Dissolve 3-bromo-5-methoxybenzaldehyde (0.48g, 2.232mmol) in toluene (26.6mL). Methyl (triphenylphosphinylene)acetate (0.746 g, 2.232 mmol) was added and the reaction was heated to reflux overnight. The reaction was cooled to ambient temperature and concentrated in vacuo. The crude material was purified by silica gel column chromatography (0 to 100% EtOAc in hexane gradient) to afford methyl 3-(E)-3-(bromo-5-methoxyphenyl)acrylate (0.580 g, 2.14 mmol , 96% yield). MS (ESI) m/z: 271/273 (M+H)+. 1H NMR (400MHz, chloroform-d) δ ppm 7.56 (1H, d, J = 16.06Hz), 7.26 (1H, s), 7.07 (1H, s), 6.95 (1H, s), 6.41 (1H, d, J = 16.06Hz), 3.82 (3H, s), 3.81 (3H, s).
Preparation[1]
3-Bromo-5-methoxybenzaldehyde was prepared as follows: Dissolve 3-bromo-5-hydroxybenzaldehyde (0.5g, 2.487mmol) in DMF (14.63mL) and cool to 0°C. NaH (0.119g, 4.97mmol) was added in three portions. The flask was immediately warmed to ambient temperature and MeI (0.933 mL, 14.92 mmol) was added and the reaction was stirred overnight. The reaction was diluted with water and partially concentrated in vacuo. The material was diluted with DCM, washed twice with water, washed with brine, dried (Na2SO4), filtered, and concentrated in vacuo. The crude material was purified by silica gel column chromatography (gradient 0 to 100% EtOAc in hexane) to give 3-bromo-5-methoxybenzaldehyde (0.488 g, 2.27 mmol, 91%). 1H NMR (400MHz, chloroform-d) δppm9.91 (1H, s), 7.58 (1H, t, J = 1.38Hz), 7.28-7.35 (2H, m, J = 2.38, 2.13, 3.01, 2.01 Hz), 3.86 (3 H, s).
Main reference materials
[1] WO2014201073 MACROCYCLIC FACTOR VIIA INHIBITORS

 微信扫一扫打赏
微信扫一扫打赏

