Background and overview[1]
3,5-Dibromoanthranilic acid methyl ester can be used as a pharmaceutical and chemical synthesis intermediate. For example, the synthesis of bromhexine hydrochloride, aliases: bromhexine, biphlegm, bisupin, chemical name: N-(2-amino-3,5-dibromobenzyl)-N-cyclohexylmethylamine salt Bromhexini hydrochloride, English name Bromhexini, is a synthetic drug obtained by structural modification of Vasicine, and its hydrochloride is commonly used. It is a white to off-white crystalline powder, easily soluble in methanol and ethanol, and slightly soluble in water. It is mainly used on the mucus-producing cells of the tracheal and bronchial mucosal glands to secrete small molecule mucin with low viscosity, thereby restoring the rheological properties of tracheal and bronchial secretion to normal, reducing phlegm and diluting phlegm. , easy to spit out.
The expectorant effect of this product is related to its promotion of ciliary movement in the respiratory mucosa and its nausea-inducing expectorant effect. It is mainly used for patients with chronic bronchitis, asthma, bronchiectasis, silicosis and other patients with white sticky phlegm that is difficult to spit out. It is an expectorant with few side effects and precise efficacy. This product can increase the distribution concentration of antibiotics in the bronchus, so the combination of the two can enhance the antibacterial efficacy of antibiotics.
Apply[1]
3,5-Dibromoanthranilic acid methyl ester can be used to synthesize bromhexine hydrochloride. The steps are as follows:
1) Preparation of reduced product: Add 200g of ethanol and 50g of calcium chloride into the reaction bottle with stirring. Stir until completely dissolved and set aside. Add 200g of ethanol into another reaction bottle and add 3,5-dibromoanthranilic acid. 100g of methyl ester, 35g of potassium borohydride, stir at room temperature for 30 minutes, start adding calcium chloride ethanol solution dropwise, control the reaction temperature at 40±2°C, complete the dripping in 2 hours, and keep the reaction for 2 hours. Cool to 25°C, control the temperature below 25°C, slowly add 200 kg of 15% hydrochloric acid, adjust the pH value to 1-2, stir for 30 minutes to maintain the pH value, cool, filter, rinse the filter cake with drinking water, and wash until medium Sex, drying. 89g of the reduced product was obtained as white or light yellow powder with a melting point of 135~143°C.
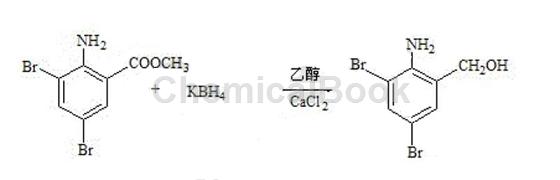
2) Preparation of crude bromhexine hydrochloride (condensation, salt formation): Put 89g of reducing material and 135.g of N-methylcyclohexylamine into the reaction bottle in sequence, and start stirring for 30 minutes. The temperature is raised to reflux. After reflux occurs, water is separated by distillation until the water separation is completed. Continue the reflux reaction for 2 hours, slowly raise the temperature to 170°C, and cool naturally. Cool the temperature to 50°C and pump in 100 g of ethanol. Then add 160 g of 15% hydrochloric acid to bring the pH to 1-2, and keep the pH unchanged for 30 minutes. The material is discharged and filtered, and the filter cake is washed with 40 g of ethanol, drained, and dried. 100g of crude bromhexine hydrochloride was obtained, with a melting point of 235~243°C.
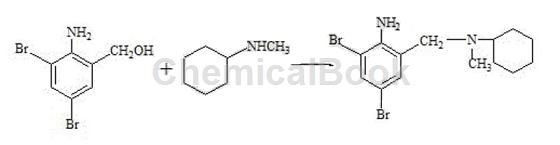
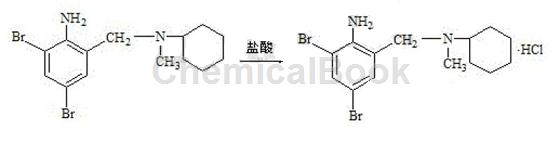
3) Refining of bromhexine hydrochloride: Add 800g of ethanol, 100g of crude bromhexine hydrochloride, and 15g of activated carbon into the reaction bottle. Start stirring, slowly increase the temperature to reflux, and maintain reflux for 30 minutes. After the reflux is completed, cool down to below 50°C, filter, rinse the filter cake with a small amount of ethanol, combine the washing liquids, concentrate the filtrate under reduced pressure to 1/2 volume, slowly cool down to 5 to 10°C, and keep it for 20 minutes. Start filtering. After the filter cake is drained, use a small amount of ethyl alcohol toRinse the wet product, continue to drain, and dry to obtain the finished product of bromhexine hydrochloride. It is white or off-white crystalline powder. Content (HPLC) ≥99.0%, melting point: 235~243℃.
Preparation [1]
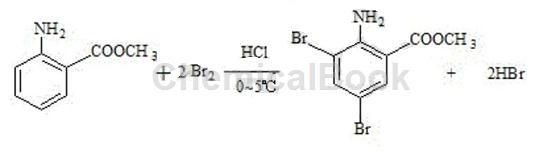
Using methyl anthranilate as the starting material, 3,5-dibromoanthranilic acid methyl ester is synthesized through bromination reaction with bromine and hydrochloric acid; the specific steps are as follows: add 240g of water into a four-neck reaction bottle, 100 g of 30% hydrochloric acid, cool to below 0-5°C, add 160g of bromine dropwise at 0-5°C (complete the dripping in 1 hour), stir and keep warm for 30 minutes, and obtain hypobromous acid for later use. Put 440g of water into another reaction bottle, start stirring, add 60g of methyl anthranilate, and cool the temperature to 0-5°C. Slowly add hypobromous acid dropwise, control the temperature below 10°C, and finish the dripping in about 2 hours. After continuing to keep warm and stirring for 1 hour, add 3000g of water, stir for 30 minutes, drain and filter, and rinse the filter cake with a large amount of drinking water. Neutral, the product is discharged and dried to obtain 118g of 3,5-dibromoanthranilic acid. It is an off-white or light yellow powder with a melting point of 80~87°C
Main reference materials
[1] CN201310540763.0 Synthesis and production method of bromhexine hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

