Application and properties[1]
Ethyl 3-nitrobenzoyl acetate can be used as an intermediate in organic synthesis and pharmaceutical research and development, and is suitable for laboratory organic synthesis and chemical and pharmaceutical synthesis processes. Ethyl 3-nitrobenzoyl acetate can be mainly used as an organic acid derivative, with a melting point of 75°C, and can participate in the synthesis of many organic reactions.
In 2014, Liu Jianqun established for the first time a HPLC-DAD method based on the Michael reaction donor molecule 3-nitrobenzoyl ethyl acetate to quickly discover the enantio-kaurene Michael reaction acceptor molecule in Rubescens rubescens. The pre-column derivatization Michael addition reaction between ethyl 3-nitrobenzoyl acetate and the anti-tumor enantiokaurene diterpene in the Rubesia rubescens extract was performed. Comparative HPLC analysis was performed on the reaction solution and the dolly grassland extract under the same conditions. According to the chromatographic peak area of enantiokaurene when the Michael addition reaction occurs, the chromatographic peak area of enantiokaurene will be reduced accordingly, and a new chromatographic peak of the Michael addition product will be generated. The original extract solution and reaction solution HPLC are determined based on the retention time, chromatographic peak shape and DAD ultraviolet spectrum. Enantiokaurene Michael-reactive receptor molecules in the map. This study provides a new method for the discovery of active components of Michael response receptor molecules.
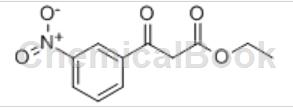
Ethyl 3-nitrobenzoyl acetate
The specific methods are as follows:
In the absence of an alkaline catalyst, the Michael addition reaction is very slow, so the author uses acetic acid to neutralize and eliminate the alkaline catalyst sodium ethoxide to terminate the reaction, and then uses HPLC to monitor the concentration changes of the reactants and products, inferring The Michael addition reaction of oridonin A and ethyl 3-nitrobenzoyl acetate.
Preparation of reactant solution: Accurately weigh 13.1 mg of oridonin A, 10.2 mg of ethyl p-nitrobenzoyl acetate, and 2.4 mg of EtONa, respectively, and place them in 25 mL volumetric flasks, and dilute to the mark with absolute ethanol. , to obtain the stock solution of each reactant.
Preparation of Michael addition reaction sample: Take 1mL of the above EtONa solution, add 10μL of glacial acetic acid to completely neutralize the catalyst, and then add 1mL of oridonin A and 1mL of 3-nitrobenzoyl ethyl acetate solution. , shake well, pass through a 0.45 μm organic microporous filter, and use it as a reaction 0-minute sample for HPLC measurement immediately. Take another 1 mL of p-nitrobenzoyl ethyl acetate solution and 1 mL of EtONa solution, mix well, and leave for 5 minutes; then add 1 mL of oridonin A, shake well, and leave at 25°C for 60 minutes to react. Then add 10 μL of glacial acetic acid to terminate the reaction. , and passed through a 0.45 μm organic microporous filter to obtain a 60-minute reaction sample, which was immediately subjected to HPLC measurement under the same conditions.
Main reference materials
[1] Liu Jianqun, Gao Junbo, Shu Jicheng, & Zhang Rui. (2014). Rapid discovery of anti-tumor enantiokaurene Michael-responsive receptor molecules of Rubescens oryzae. Strait Pharmacy (7).

 微信扫一扫打赏
微信扫一扫打赏

