Background and overview[1][2]
1-Methyl-p-toluenesulfonylmethyl isonitrile is also known as -((1-isocyanoethyl)sulfonyl)-4-toluene; 1-methyl-1-toluenesulfonylmethylisonitrile Cyanide; 1-methyl-1-tosylmethylisobutyl ester, Benzene, 1-[(1-isocyanoethyl)sulfonyl]-4-methyl-; 1-Tosylethylisocyanide; a-Tosylethylisocyanide; is a chemical in organic synthesis , especially the synthesis of five-membered organic heterocycles and the widely used intermediates, which have been used in the synthesis of drugs and other fine chemical products. However, the existing preparation method of 1-methyl-p-toluenesulfonylmethylisonitrile has a very low yield. Since it is mixed with water, it is generally not recovered. When prepared in large quantities, it increases the cost and pollutes the environment. Cheap or low-cost methods should be considered. Easily recyclable solvent replacement.
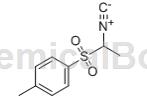
1-Methyl-p-toluenesulfonylmethylisonitrile
Preparation[2]
Preparation method of 1-methyl-p-toluenesulfonylmethylisonitrile:
Step 1: In a reaction kettle equipped with a stirrer, add N-p-toluenesulfonylmethylformamide, dimethylethyl chloride, anhydrous ether and acetonitrile while stirring, and dissolve the stirring suspension Cool to -3°C in an ice-salt bath, add dropwise the mixed solution of ethanol and n-ethane, and complete the dropwise addition within 35m at a constant speed, the white suspension gradually turns brown;
Step 2: Keep the above brown suspension at 0°C and stir for 40 minutes. Add ice water while stirring. Filter out the dark brown precipitate from the brown suspension. Dissolve the dark brown precipitate in butylbenzene at 68°C and add activated carbon. , stir for 10 m and then filter. Add petroleum ether to the solution while vortexing. After 30 m, filter out the precipitate with suction and dry it in a vacuum dryer for 7 hours to obtain a light brown colorless solid product.
Main reference materials
[1] Ding Chengrong, Zhang Chaoyang, Wang Xiangang, Xiao Jun, Yan Yunbing, & Zhang Guofu. (2012). Synthesis of p-toluenesulfonylmethyl isonitrile. Pesticides, 51(12), 869-871.
[2] Jiang Shuyan, Zhao Yan, & Han Yuding. (1997). Study on reaction conditions for the synthesis of p-toluenesulfonylmethyl isonitrile. Shandong Chemical Industry (2), 16-18.
[3] Wang Yanan, Liu Shiwei, Li Lu, & Yu Shitao. (2016). Synthesis study of p-toluenesulfonylmethylisonitrile. Chemical Technology, 24(1), 37-40.

 微信扫一扫打赏
微信扫一扫打赏

