Background and overview[1][2]
Organic compounds containing an isocyanate group in their molecular structure are generally called isocyanates. Organic isocyanates are important organic synthesis intermediates and are widely used in pesticides, dyes, coatings, leather polishes, adhesives, artificial leather, polyurethane waterproof materials, potting materials, soft and hard foams, elastomers and acrylic urethanes. It has a wide range of applications in molecular materials. The production of isocyanates has attracted the attention of countries around the world, and its output is increasing year by year. Among them, the application degree of polyurethane and other plastic products has become one of the indicators to measure a country’s comprehensive national strength and modernization level. The development of polyurethane and its products is closely related to the development of isocyanate raw materials. Therefore, the research and development of isocyanates is of extremely important strategic significance.
Currently, most isocyanates at home and abroad use the traditional phosgene method. This method has the disadvantages of using the highly toxic raw material phosgene, producing a large amount of highly corrosive hydrochloric acid, residual chlorine affecting product quality and waste emissions, etc., and will eventually Be eliminated. The chemical industry at home and abroad is studying the cleaning process for the production of isocyanate. The main development trend is the two-step method. The first step is the synthesis of carbamate, and the second step is the thermal decomposition of carbamate. Regarding the synthesis of carbamates, there are nitrobenzene reductive dialization methods, aromatic amine oxidative dialization methods, and aromatic amine methoxy dialization methods. Meta-toluene isocyanate is an important isocyanate, mainly used in the manufacture of polyurethane materials, and can be substituted or used in some situations. In addition, m-toluene isocyanate can also be used as an important pharmaceutical and pesticide intermediate.
Structure
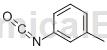
Apply[1][2]
Isocyanate is an important type of organic synthesis intermediate, which can be widely used in the synthesis of polymer materials such as polyurethane, coatings, dyes and pesticides. The isocyanate product series mainly includes toluene diisocyanate, diphenylmethane diisocyanate and phenyl isocyanate. As the scale of the polyurethane industry expands year by year, the development of isocyanate production technology has attracted the attention of scholars from various countries. Meta-toluene isocyanate is an important isocyanate, mainly used in the manufacture of polyurethane. It can replace or to a certain extent, thus alleviating the shortage of isocyanate at home and abroad.
Moreover, it is also an important pharmaceutical and pesticide intermediate. Ruyouyanjiu has developed a skin wound dressing with a double-layer structure based on polysiloxane supramolecular elastomer and its preparation method. The method steps are as follows: amino-terminated polysiloxane reacts with hexamethylene diisocyanate to obtain a polysiloxane supramolecular elastomer used as a flexible substrate layer; carboxyl-terminated polysiloxane is successively reacted with monofunctional and The difunctional primary amine compound reacts to obtain a siloxane oligomer containing secondary amine groups; the siloxane oligomer is then reacted with m-toluene isocyanate and hexamethylene diisocyanate successively to obtain a poly(polymer) used as an adhesive layer. Silicone supramolecular elastomer; and then prepare a double-layer polysiloxane supramolecular elastomer film dressing through hot pressing and cold pressing molding processes.
This film dressing has good air permeability and water absorption. Using it as a chronic wound dressing is beneficial to the growth and regeneration of wound tissue and accelerates wound healing. This method uses polysiloxane as raw material, has no cytotoxicity and skin irritation, and has good biocompatibility.
Preparation [1]
Method 1: 1) Synthesis operation: Before the reaction, add accurately weighed m-toluidine, catalyst sodium methoxide and solvent methanol into a four-necked flask, replace the air in the four-necked flask, and heat in an oil bath under stirring conditions Raise the temperature to about ℃, reflux, and start timing. After the reaction was completed, the reaction solution was collected after cooling to room temperature. 2) Post-treatment operation: Use hydrochloric acid to neutralize the reaction solution to about 20%, and then filter it. The purpose of adding hydrochloric acid is to convert the catalyst sodium methoxide dissolved in methanol into white sodium chloride precipitate.
Pour the obtained filtrate into a beaker and perform vacuum distillation until no distillate appears. Stop distillation and cool. If there is solid precipitated in the flask, if there is still too much liquid, filter it to obtain a light yellow solid. , recrystallized with absolute ethanol to obtain white needle-like crystals, which is the methyl m-toluene carbamate product of this experiment.Heavy.
Method 2: 1) Synthesis operation: The reaction of m-toluidine and dimethyl carbonate to synthesize m-toluidine methyl carbamate is carried out in a stainless steel high-pressure reactor. Before the reaction, add accurately weighed m-toluidine and catalyst into the reaction kettle, replace the air in the kettle, heat and raise the temperature under stirring conditions, and start timing after the temperature becomes constant. 2) Post-processing operation: After the reaction is completed, pass the cooling water through the reaction kettle to room temperature, open the pressure relief valve to relieve the pressure to zero, then open the autoclave and take out the reaction solution in a beaker.
Because the high-temperature reaction solution needs to be lowered to room temperature, some products will precipitate from the reaction solution. In order to fully dissolve this part of the product and the solids remaining in the washing kettle, pour it into the kettle and wash the kettle. Combine the reaction solution poured in the previous step with the reaction solution in the previous step, and slowly heat until the product dissolves. Immediately filter under reduced pressure in a hot funnel to remove the solid residue containing the catalyst, and collect the residue for subsequent processing. Pour the obtained filtrate into a flask and perform vacuum distillation until no liquid flows out. Stop distillation and cool. If solid precipitates in the flask, weigh the crude product. Finally, the crude product was purified by recrystallization with absolute ethanol to obtain a white crystalline solid, which was weighed.
Main reference materials
[1] Research on green synthesis process of m-toluene isocyanate
[2] CN201610365141.2 A skin wound dressing with a double-layer structure based on polysiloxane supramolecular elastomer and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

