Background and Overview
5-Bromo-2-trifluoromethoxybenzaldehyde is an aldehyde organic compound that can be used in organic synthesis.
Preparation[1]
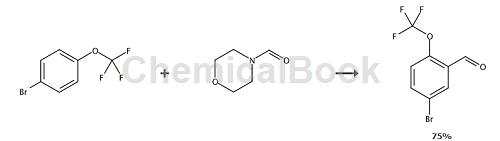
To a solution of diisopropylamine (3.5 mL) in THF (50 mL), add a solution of 1.6 mol/L n-butyllithium in hexane (15 mL) at 0°C. After the reaction mixture was cooled to -78°C, 1-bromo-4-(trifluoromethoxy)benzene (6.0 g) was added, and the mixture was stirred for 2 hours. Then, morpholine-4-carboxaldehyde (8.6 g) was added to the reaction mixture, and the mixture was concentrated to dryness under reduced pressure. Water was added to the residue, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated aqueous sodium bicarbonate solution and then brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography (solvent gradient; 0 → 30% ethyl acetate/hexane) to afford 5-bromo-2-(trifluoromethoxy)benzaldehyde (5.04 g, 75%) as free Oily color. Yield 5.04g, 75%.
Apply [2]
5-Bromo-2-trifluoromethoxybenzaldehyde can be used to prepare cyclopentanedione derivative herbicides.
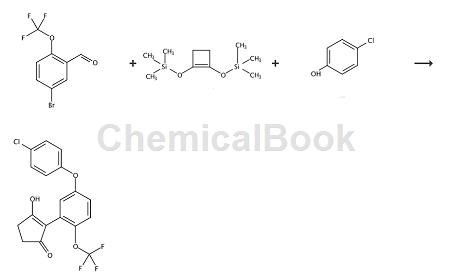
Step 1: Preparation of 2-(5-bromo-2-trifluoromethoxyphenyl)cyclopentane-1,3-dione
To a solution of 5-bromo-2-trifluoromethoxybenzaldehyde (2.0g, 7.43mmol) in anhydrous dichloromethane (40ml) at room temperature, add boron trifluoride etherate (1.13 ml, 8.92 mmol), then 1,2-bis(trimethylsiloxy)cyclobutene (2.86 ml, 11.2 mmol) was added. The mixture was stirred at room temperature for 23 hours, then distilled water (1.2 ml) and additional boron trifluoride etherate (14.1 ml, 112 mmol) were added. After stirring at room temperature for 24 hours, the reaction mixture was then quenched with saturated aqueous ammonium chloride solution (50 ml) and extracted with dichloromethane (2 x 50 ml). The crude product was extracted into 0.5M aqueous potassium carbonate solution (x3), and the aqueous phase was acidified to pH 1 with concentrated hydrochloric acid. Final extraction with dichloromethane (x3) followed by washing with brine and drying over magnesium sulfate. Filtration and concentration in vacuo gave the crude product, which was purified by preparative reversed phase HPLC to give 2-(5-bromo-2-trifluoromethoxyphenyl)cyclopentane-1,3-dione.
Step 2: Preparation of 2-[5-(4-chlorophenoxy)-2-trifluoromethoxyphenyl]cyclopentane-1,3-dione
To a mixture of 4-chlorophenol (0.473g, 3.69mmol), cesium carbonate (0.521g, 1.48mmol), copper triflate (0.013g, 0.04mmol) and powdered molecular sieve (0.400g) A solution of 2-(5-bromo-2-trifluoromethoxyphenyl)cyclopentane-1,3-dione (0.249 g, 0.74 mmol) in dry toluene (3.5 ml) was added. The mixture was flushed with nitrogen and heated under microwave irradiation at 160°C for 1 hour and then at 170°C for an additional hour. After cooling to room temperature, the crude product was partitioned between dichloromethane (5 ml) and 2M hydrochloric acid (5 ml), the organic phase was separated and concentrated in vacuo. The residue was then purified by silica gel flash column chromatography (ethyl acetate/isohexane eluent) to give 2-[5-(4-chlorophenoxy)-2-trifluoromethoxyphenyl]cyclopentane -1,3-diketone, white��Solid. 1H NMR (unless otherwise stated, CDCl3) δ7.33-7.29 (m, 3H), 7.13 (d, 1H) , 7.00-6.95 (m, 3H), 2.68 (s, 4H).
Main reference materials
[1] U.S. Pat. Appl. Publ., 20070149570, 28 Jun 2007
[2] PCT Int. Appl., 2010102848, 16 Sep 2010

 微信扫一扫打赏
微信扫一扫打赏

