Background and overview[1]
3-Amino-3-(2-nitrophenyl)propionic acid can be used as a pharmaceutical synthesis intermediate. If 3-amino-3-(2-nitrophenyl)propionic acid is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water, if any If you feel discomfort, seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation [1]
The preparation of 3-amino-3-(2-nitrophenyl)propionic acid is as follows:
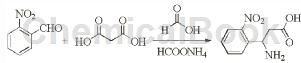
The specific steps are as follows: stir o-nitrobenzaldehyde (20.4 g, 0.135 mol), formic acid (20.3 mL, 0.539 mol) and malonic acid (18.3 g, 0.176 mol) at 45 °C for half an hour, and then Ammonium formate (21.3 g, 0.338 mol) was added to it, the reaction temperature was raised to 70°C and stirred for one hour, then stirred at 95°C for four hours, then concentrated hydrochloric acid (50 mL) was added and the temperature was maintained for one hour. , cool, add 25mL water, wash twice with ethyl acetate (2X25mL), adjust the pH of the water phase to 4.2 with 50% potassium hydroxide solution, filter the precipitated solid, and dry it under vacuum to obtain 18.33 g of yellow solid, yield 64.6%.
Apply[1]
3-Amino-3-(2-nitrophenyl)propionic acid can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
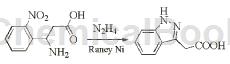
The specific steps are: Dissolve 3-amino-3-(2-nitrophenyl)propionic acid (15 g, 71.4mmol) in 5% sodium hydroxide solution (85 mL) and 85% hydrazine hydrate (5mL) in the mixed solution, the reaction was heated to 80 ° C, and then Raney nickel (2 × 25 mg) was carefully added. After the reaction for half an hour, it was cooled, and the pH was adjusted to 2 with 6W hydrochloric acid. The precipitated solid was filtered and vacuum dried to obtain Yellow solid 6.86 g, yield 54.5%.
Main reference materials
[1] WO2013071880 Nitrogen-containing paracyclic compounds as CRTH2 antagonists

 微信扫一扫打赏
微信扫一扫打赏

