Background and overview[1]
2-Chloro-4-bromo-5-fluorotoluene can be used as a pharmaceutical synthesis intermediate. If you inhale 2-chloro-4-bromo-5-fluorotoluene, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable. ; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation [1-2]
2-Chloro-4-bromo-5-fluorotoluene can be used as a pharmaceutical synthesis intermediate if the following reaction occurs:

The specific steps are: add a mixture of 2-chloro-4-bromo-5-fluorotoluene (5.0g, 23mmol), copper(I) cyanide (4.0g, 45mmol) and copper(I) iodide (8.6 g, 45 mmol) was dissolved in NMP (50 mL) and heated at 140°C overnight. After cooling to room temperature, the mixture was filtered, the filtrate was diluted with water (100 mL), and extracted with ethyl acetate (100 mL × 3). The combined organic layers were washed with water (100 mL) and dried over NaSO. The solvent was removed and the residue was purified by SGC (eluting with petroleum ether/EtOAc = 10/1) to give 5-chloro-2-fluoro-4-methylbenzonitrile (1.7 g, 45% yield) as white solid.
In addition, 2-chloro-4-bromo-5-fluorotoluene can also undergo the following reactions:
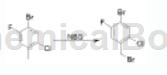
The specific steps are: combine 2-chloro-4-bromo-5-fluorotoluene (10.0g, 44.75mmol, 1.0 equivalent), NBS (7.9g, 44.75mmol, 1.0 equivalent) and AIBN (736mg, 4.48mmol) The mixture was heated to reflux in 0.1 L eq. CCL (250 mL) and stirred under N2 for 3 h. Concentrate the mixture. The residue was purified by column chromatography (PE-PE/EA = 30/1, v/v) to give 1-bromo-4-bromomethyl-5-chloro-2-fluoro-benzene (7.5 g, 56%), It is oily.
Main reference materials
[1] US20160009667 Substituted benzoxazoles and methods of use thereof
[2] WO2015103317 THERAPEUTIC INHIBITORY COMPOUNDS

 微信扫一扫打赏
微信扫一扫打赏

