Physical and chemical properties and structure[1]
Fungicides occupy a considerable position among pesticides. However, the currently commonly used fungicides are mainly traditional dithiocarbamates, benzimidazoles and triazoles. Acetophenone oxime is a type of benzoheterocyclic compound containing adjacent oxygen atoms and nitrogen atoms. It has high biological activity and pharmaceutical properties and is commonly used as pesticides and insecticides.
Acetophenone oxime derivatives have good bactericidal activity and control effect on some common diseases of vegetable and fruit crops such as powdery mildew, anthracnose, sclerotinia, etc., especially the control effect on powdery mildew is better. The dosage is low, the effect is good, and the preparation is convenient. It can reduce agricultural costs and improve environmental protection. Acetophenone oxime derivatives are expected to become a novel and effective agricultural fungicide, which can be used to replace those that have developed severe resistance and are currently on the market. The usage amount is several times the original amount of traditional agricultural fungicides.
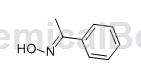
Acetophenone oxime
Apply[3]
Acetophenone oxime can be used in the preparation of acetophenone–O–ethyl oxime ether
Add 3.645g (27mmol) acetophenone oxime into the reaction bottle, dissolve it in 10mL absolute ethanol, cool to 0℃~5℃ while stirring, and add 1.188g (29.7mmol) hydroxide dropwise at this temperature Sodium saturated solution, then add dropwise a mixture of 2.943g (27mmol, 2.0ml) bromoethane and 5ml absolute ethanol, finish dropping in about 0.5h, stir at room temperature overnight, extract 3 times with ethyl acetate, anhydrous sodium sulfate Dry, remove the solvent and concentrate, then use column chromatography with a 10:1 mixture of petroleum ether and ethyl acetate to obtain light yellow liquid 28 with a yield of 62%.
Preparation[2]
Add 6.4ml (55mmol) acetophenone, 6g (86mmol) hydroxylamine hydrochloride, 4ml newly prepared distilled water and 20ml 95% ethanol into the reaction bottle, add 11g (275mmol) solid sodium hydroxide in batches in an ice bath, this When the solution becomes a white turbid liquid, heat it up and reflux for five minutes. After cooling, pour the solution into a beaker containing 30 ml of concentrated hydrochloric acid and 200 ml of water to obtain a white solid acetophenone oxime with a melting point of 58°C to 60°C. The melting point in the literature is 55℃~56℃.
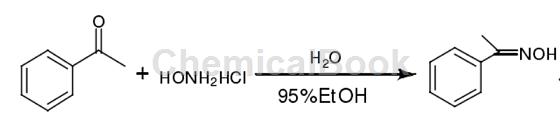
Main reference materials
[1] Du Guiying, & Zhang Qingquan. (1997). Study on the rearrangement reaction of acetophenone oxime under the action of ultrasound. Journal of Hebei University (Natural Science Edition) (1), 38-40.
[2] Yu Linhua, Li Xuanhai, Li Linyan, Zeng Zhongxian, Fan Minguang, & Xu Shengming et al. (2014). Synthesis of 2-hydroxy-5-teroctylacetophenone oxime. Hydrometallurgy (4), 292-296.
[3] Li Xiaojun, Feng Cheng, Li Chunying, & Chen Wei. (2007). Synthesis of extraction agent 2-hydroxy-5-nonylacetophenone oxime. Tianjin Chemical Industry, 21(1), 21-22.

 微信扫一扫打赏
微信扫一扫打赏

