Overview[1]
3-Chloro-2-methylphenylboronic acid, also known as (3-chloro-2-methyl)phenylboronic acid; 2-methyl-3-chlorophenylboronic acid, is a white solid powder and is used A very wide range of chemical intermediates, widely used in the synthesis of medicines and other fine chemical products.
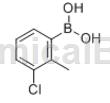
3-Chloro-2-methylphenylboronic acid
Apply[3]
3-Chloro-2-methylphenylboronic acid can be used in the preparation of 3-chloro-2-methylphenylboronic acid pinacol ester:
Under nitrogen protection, add (1,5-cyclooctadiene)(methoxy)iridium(I) dimer (20mg, 0.03mmol) and 2,2′-dipyridine to the double-necked flask. (9.37mg, 0.06mmol), pinacol diborate (2.35g, 9.26mmol), then add 15mL tetrahydrofuran, stir at room temperature for 10min, the reaction system turns dark green, then add 3-chloro-2-dimethoxy Toluene (1.88g, 12.35mmol) was heated to 70°C and refluxed for 8 hours. After the reaction was completed, the reaction mixture was concentrated and spun to dryness, and then separated by silica gel chromatography column to obtain 3-chloro-2-methylphenylboronic acid (2.23g, 65%).
3-Chloro-2-methylphenylboronic acid is an important organic synthesis intermediate and is widely used in the fields of drug synthesis and basic scientific research. For example, it is used in the synthesis of topoisomerase II inhibitor PF-03941275, AMPA receptor potentiator LY451395, non-peptide growth hormone secretagogue SM-130686, and a bradykinin antagonist developed by Merck. aryl pinacol esters.
Preparation[2]
(1) Acetal preparation: Under nitrogen protection, add 40g 3-chloro-2-methylbenzaldehyde, 25g ethylene glycol, 250ml toluene, and 4g p-toluenesulfonic acid into a 1000mL three-necked flask, and heat to reflux to separate the water. After the reaction, it was washed with a 30% potassium carbonate solution, dried, desolvated, and distilled to obtain 40 g of pure 3-chloro-2-methylbenzaldehyde ethylene glycol acetate, with a yield of 82%.
(2) Lithiation: Under nitrogen protection, add the 2-methyl-5-bromobenzaldehyde ethylene glycol prepared above into a 1000mL dry three-necked flask, add 400ml tetrahydrofuran, and cool to -80°C , then add 96 ml of n-butyllithium dropwise, keeping -80°C during the dropwise addition. After the dropwise addition is completed, perform a heat preservation reaction, control the reaction temperature to -80°C, and prepare a phenyllithium compound intermediate.
(3) Boration: The temperature of the obtained phenyllithium compound is cooled to -80°C, and 60g of tributyl borate liquid is added dropwise to the phenyllithium solution prepared above. After the dropwise addition is completed, the insulation reaction is carried out. The insulation reaction temperature was -80°C. After the reaction is completed, add hydrochloric acid with a mass concentration of 10% to the reaction system and stir. During the stirring process, the acetal is automatically deprotected. The pH value of the reaction solution is adjusted to 1 to 2, and then left to stand for layering. After extraction, the organic layers are combined. Dry, remove the solvent, add water and then spin it to dryness to remove the solvent. Spin off the generated butanol to obtain 26.4g of crude 3-chloro-2-methylphenylboronic acid, with a yield of 80%.
(4) Purification: Add 26.4g of crude 3-aldehyde-4-methylphenylboronic acid into a 500ml Erlenmeyer flask, add ethyl acetate to heat melt, then add 100ml of petroleum ether, cool, crystallize and centrifuge to obtain 23.07g 3-Chloro-2-methylphenylboronic acid product, yield 70%.
Main reference materials
[1] Hong Xiaobo, Zeng Kui, Lei Jinxiu, Ping Li, & Yu Lushan. (2018). Determination of 4-chloro-2-fluoro-3-methoxyphenylboronic acid in rat plasma by Lc-ms/ms Research on its toxicokinetics. Chinese Modern Applied Pharmacy (1).
[2] Li Huanhuan, Gai Yongjian, Gao Qing, & Zhao Jie. (2016). Study on the acute toxicity of 4-chloro-2-fluoro-3-methoxyphenylboronic acid. Practical Preventive Medicine, 23(2 ), 226-228.
[3] J. Oppenheimer. (2014). Method for separating 4-chloro-2-fluoro-3-substituted-phenylboronic acid. CN103814034A.

 微信扫一扫打赏
微信扫一扫打赏

