Background and overview[1]
1-Methyl-2-formylbenzimidazole can be used as a pharmaceutical synthesis intermediate. If 1-methyl-2-formylbenzimidazole is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell. ; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 1-methyl-2-formylbenzimidazole is divided into two steps, as follows:
Step 1: Dissolve N-methylbenzene-1,2-diamine (13.5g, 85.4mmol) and tartaric acid (6.4g, 42.6mmol) in 4NHQ aqueous solution (100mL), and reflux the solution Stir overnight. After cooling to room temperature, the precipitate was collected by vacuum filtration and redissolved in water (200 mL), and the pH of the resulting mixture was adjusted to 8 with aqueous NH4OH solution. The precipitate was collected by vacuum filtration and dried to obtain 6.86 g of the desired product 1,2-bis(1-methyl-1H-benzo[d]imidazol-2-yl)ethane-1,2-diol, which was It was used in the next step without further purification. LC-MS: m/z323 (M+H+).
Step 2: Add 1,2-bis(1-methyl-1H-benzo[d]imidazol-2-yl)ethane-1,2-diol (4.9g, 15.2mmol) and NaIO4 ( 3.3g, 15.2mmol) solution (150mL) was added dropwise with 3NH2SO4 aqueous solution (18mL). After stirring at ambient temperature overnight, the mixture was adjusted to pH 8 with aqueous Na2CO3 solution. The precipitate was collected by vacuum filtration and washed with water and ethanol. The solid was dried to obtain 3.11 g of the desired product 1-methyl-2-formylbenzimidazole. LC-MS: m/z161 (M+H+).
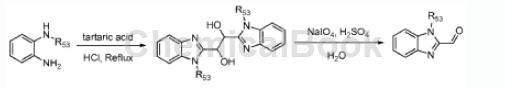
(R53=-CH3)
Main reference materials
[1] WO2011150156 HETEROARYLCOMPOUNDSANDMETHODSOFUSETHEREOF

 微信扫一扫打赏
微信扫一扫打赏

