Background and overview[1]
4-(4-Methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene can be used as a pharmaceutical synthesis intermediate. If 4-(4-methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene is inhaled, move patient to fresh air; if skin contact occurs, remove contaminated clothing Clothes, rinse skin thoroughly with soap and water, and seek medical attention if you feel unwell; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, and do not induce vomiting. Seek medical attention immediately.
Structure
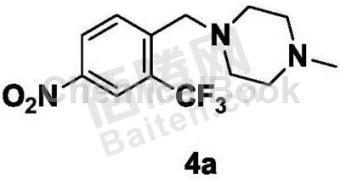
Preparation[1]
The preparation of 4-(4-methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene is divided into the following two steps:
Step 1: In a 100mL two-necked round-bottomed flask, add 1-methyl-4-nitro-2-(trifluoromethyl)benzene (compound 1) (2g, 9.76mmol), NBS (1.91 g, 10.73mmol), BPO (0.47g, 1.95mmol), seal the system, evacuate, and protect with N2, and then use a 20mL syringe to inject the solvent CCl4 (20mL×2). Warm the system to 85°C, stir for 5 hours, add 20 mL of saturated NaHCO3 solution to quench the reaction, extract with dichloromethane (20 mL × 2), combine the organic phases, dry over anhydrous Na2SO4, filter, and concentrate under reduced pressure to obtain crude product without purification. , directly vote for the next step.
Step 2: In a 50mL round-bottomed flask, add crude compound 2 (2g, 7.04mmol), compound 3 (actually compound 3a or 3b, for simplicity, there is no need to distinguish between compound 3a or 3b) Called compound 3, other compounds can also be treated similarly) (14.08mmol), K2CO3 (1.95g, 14.08mmol), 30mLTHF, stir at room temperature for 4h, and detect the reaction by TLC. After the reaction is completed, concentrate under reduced pressure to remove THF, add 20 mL of saturated NaHCO3 solution, extract with dichloromethane (30 mL×2), combine the organic phases, dry over anhydrous Na2SO4, filter, concentrate under reduced pressure, and purify by column chromatography (CH2Cl2: CH3OH=30:1, ammonia: 0.5%) to obtain compound 4, yield: 60%-75%.
Apply[1]
4-(4-Methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
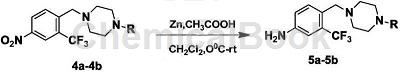
Take a 100mL two-necked round-bottomed flask, add 4-(4-methylpiperazine-1-methylene)-3-trifluoromethyl-1-nitrobenzene (3.30mmol), zinc powder (7.11 g, 108.81mmol), dichloromethane 25mL. After the system is cooled to 0°C, CH3COOH (1.58g, 26.34mmol) diluted in 10 mL of methylene chloride is slowly added dropwise to the system using a dropping funnel. After the dripping is completed, the mixture is stirred at 0°C for 1 hour and then moved to Stir at room temperature for 4 h, and check the reaction progress with TLC. After the reaction, filter the zinc powder, wash the filter cake with dichloromethane, combine the organic phases, wash with 20 mL :1, ammonia: 0.5%) to obtain compound 5, yield: 62%-70%.
Main reference materials
[1] CN201611248003.2 Preparation and application of triazole compounds

 微信扫一扫打赏
微信扫一扫打赏

